Abstract
Background
Abdominal asymmetry from fascial laxity is a common occurrence after harvesting the rectus abdominis muscle for use in pedicled and free flap breast reconstruction. Many techniques have been described to prevent or eliminate these abdominal bulges, but recurrences are still seen.
Summary
We present a unique option for eliminating an abdominal bulge after transverse rectus abdominis myocutaneous (TRAM) flap breast reconstruction. Our technique involves two separate layers of mesh, one placed in an onlay fashion over the anterior rectus sheath, after fascial imbrication, and the second mesh placed laparoscopically in the preperitoneal space. The patient we present previously developed a recurrent abdominal bulge after a TRAM. Our repair has prevented a recurrence at 10 months postoperatively.
Conclusion
Abdominal asymmetry from fascial laxity after TRAM flap breast reconstruction causes aesthetic concerns for patients and often causes abdominal wall discomfort. Traditional repair involves fascial imbrication and reinforcement with an onlay mesh. Despite this repair some patients develop a recurrence in the bulge. Our technique of eliminating this bulge and reinforcing the repair with two layers of the mesh, one onlay and the second placed laparoscopically in the preperitoneal space, is novel and has proven successful against recurrence in our patient.
Key Words
Transverse rectus abdominis myocutaneous flap, abdominal bulge, abdominal asymmetry, onlay mesh, preperitoneal mesh
Case Description
Removal of a portion of the rectus abdominis muscle in pedicled and free transverse rectus abdominis myocutaneous (TRAM) flaps results in alteration of the abdominal wall functional mechanics, which often results in the development of laxity and an associated bulge. Abdominal bulge rates have been reported to be as high as 20 percent in patients who have undergone breast reconstruction utilizing the rectus muscle.1
A large number of publications exist that address various efforts to minimize abdominal wall morbidity after abdominally-based autologous breast reconstruction. Inclusion of mesh over primary repair or imbrication of the fascia has been shown to decrease bulge and hernia rates.2-4 Use of synthetic, instead of biologic mesh, also decreases the risk of a patient developing an abdominal bulge.2 Muscle-sparing TRAM or deep inferior epigastric perforator free flap breast reconstruction has been shown to significantly reduce the risk of developing a postoperative bulge compared to the pedicled or free TRAM techniques; however, these techniques result in longer operative times and higher rates of fat necrosis and partial or total flap loss.1 Additionally, despite preservation of the rectus muscle at the donor site, studies have shown that denervation of the rectus muscle during dissection can also result in the development of a postoperative bulge.5
After a bulge develops, management and recurrence prevention is difficult because no definitive fascial defect exists. In our practice, patient dissatisfaction with the unsightly appearance of the abdominal contour abnormality, in addition to pain and functional core weakness are the strongest motivators for reoperation. Various techniques have been described for elimination of abdominal bulges. Primary suture plication with or without mesh reinforcement is the simplest documented technique for repair, however, recurrence rates between 20.9 and 69 percent have been reported.6,7 Specialized techniques, such as the internal oblique repair described by Kroll et al., could further lower the recurrence rate but are more technically challenging and require incisions through fascia, which could result in the development of a true hernia. Lee et al. described the placement of mesh laparoscopically in nine patients to repair bulges associated with abdominal wall laxity after DIEP and TRAM flap breast reconstruction. In this repair technique, they placed one piece of intraperitoneal mesh laparoscopically to reinforce the abdominal wall.8
Souto et al described the placement of two layers of synthetic mesh in separate fascial planes to correct abdominal wall defects secondary to pedicled TRAM breast reconstruction.9 The deepest mesh was placed in the preperitoneal space and the second mesh was placed between the external and internal oblique muscles. The reinforcement of multiple layers of the abdominal wall with mesh seems to add additional protection against recurrence, however, this technique was described in patients with hernias and not bulges with intact fascia.
We present a novel technique to repair abdominal wall bulges that develop after abdominally-based breast reconstruction. Our technique involves two layers of mesh, one placed in an onlay fashion after imbrication of the fascial laxity and the other placed laparoscopically in the preperitoneal space as a sublay. This unique technique provides a strong, multiple-layered repair while at the same time minimizing procedure morbidity without disruption of the intact fascia. The repair was performed in a patient who had undergone a pedicled TRAM and developed a recurrent bulge after prior attempted repair.
Our patient is a 70-year-old female who was diagnosed with invasive ductal carcinoma of the left breast. She subsequently had a left mastectomy with sentinel lymph node biopsy and adjuvant chemoradiation therapy. She was interested in pursuing breast reconstruction and due to her radiation therapy it was recommended that she consider autologous reconstruction from the abdomen. She was in agreement and opted for left breast reconstruction with a right pedicled transverse rectus abdominis myocutaneous (TRAM) flap. During this operation, she had primary fascial closure of the anterior rectus sheath. Strattice mesh (Acelity, San Antonio, TX) was used to reinforce the fascial closure.
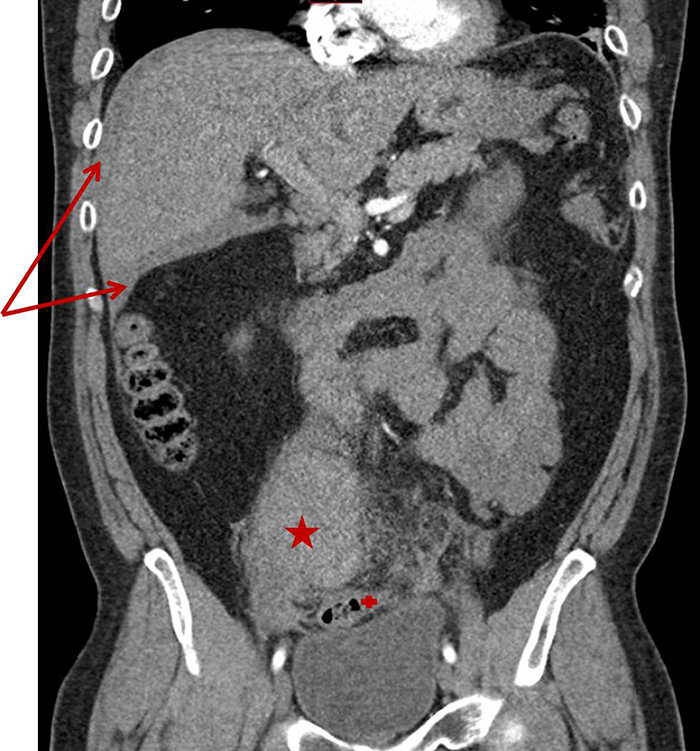
Two months postoperatively, the patient developed cellulitis of the abdomen, which required hospital admission and a course of intravenous antibiotics. At her six month follow-up visit she identified a bulge in her right lower quadrant when standing upright (Figure 1). This bulge would disappear when supine. There was no palpable fascial defect on examination. In subsequent follow-up visits the bulge was noted to be enlarging and becoming increasingly more painful to the patient.