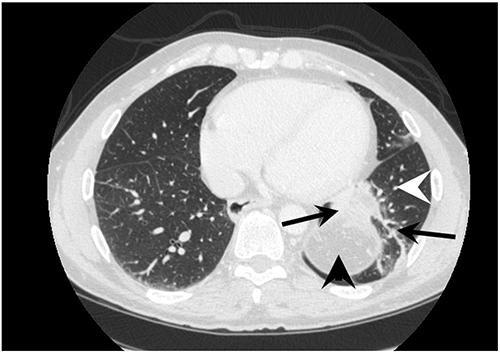
Figure 2. Postoperative (four-year) CT scan in the porto-venous phase demonstrating collateral circulation (black arrows) between the spleen (black arrowhead) and lung (white arrowhead)
Discussion
This case illustrates that variceal hemorrhage not amenable to endoscopic treatment can be controlled with a modified Sugiura procedure. Additionally, long-term decompression of the portosplenic system may be achieved by creating a surgical shunt between the parenchyma of the spleen and left lung. Ten percent of portal hypertension is caused by non-cirrhotic diseases.6 These can be hereditary, including hypercoagulable disorders like Factor V Leiden mutation, Protein C or S deficiency, or Prothrombin gene mutation, or acquired, secondary to malignancy, abdominal surgery, or blunt abdominal trauma among others.2,6 Chronic mesenteric thrombosis may lead to portal hypertension when it involves the portal and splenic veins, and may present with variceal bleeding.3,4 In this case, the patient had a prothrombin gene mutation, complete PSM thrombosis with portal hypertension and variceal bleeding. Typical management of variceal hemorrhage aims to control variceal bleeding and prevent re-bleeding. First-line therapy includes pharmacologic and endoscopic intervention, with emergency surgical shunting as a last resort.2,3,6,7 In therapy refractory hemorrhage, initial mechanical compression of gastroesophageal varices should be performed.1,8
In this case, first-line therapy failed. A Sengstaken-Blakemore tube was placed to temporarily stabilize the patient. Since the thrombosis affected all splanchnic veins, emergency TIPS or common surgical shunt procedures were not an option; therefore, a modified Sugiura procedure was performed to stop bleeding. A similar modified Sugiura procedure described by Ginsberg et al. consisting of esophageal devascularization, vagotomy, and Nissen fundoplication had a two-year rebleed rate of 25 percent when done in cirrhotic patients.9 In addition, a splenopneumopexy was completed to relieve the PSM hypertension and to induce new collateral formation between the spleen and left lung.10 Splenopneumopexy was initially described in a case series of 15 patients with Budd-Chiari syndrome. The series demonstrated reduced splenic pulp pressures after surgery (from a mean of 24.8 mm Hg to a mean of 19 mm Hg), and collateral circulation through the spleen and lung w as shown, via splenoportography with 85Kr tracing, to carry 47.5 percent of total portal flow within one month after the procedure. Moreover, all but one patient had resolution of ascites, and the two-year survival rate was 73 percent.10 Although Budd-Chiari is routinely managed currently by portosystemic shunting, this original series suggested the effectiveness of long-term splanchnic decompression by shunting blood via the spleen to the lungs. In this case, we did not assess initial and repeated splenic pulp pressures like the above-mentioned study. However, a dynamic CT scan was performed four years after treatment and the extent of varices and collaterals was investigated. The CT demonstrated collateral circulation through the splenic-pulmonary interface, reduction in spleen size, and resolution of ascites. Our patient is now four years postsurgery without further bleeding episodes.
This case illustrates that splenopneumopexy may be beneficial when added to the Sugiura procedure in cases of extensive PSM thrombosis that is not manageable by repeated banding of gastroesophageal varices alone. It combines two surgical procedures for short-term (modified Sugiura procedure) and long-term (splenopneumopexy) alleviation of portal hypertension and variceal bleeding, and it may result in excellent clinical outcomes.
Conclusion
Splenopneumopexy in addition to the Sugiura procedure allows for the development of portosystemic collaterals in patients with no other portosystemic shunt options. It should be considered by surgeons who deal with difficult-to-treat portal hypertension where the PSM system is occluded.
Lessons Learned
Emergent esophageal bleeding is not always caused by cirrhosis and may be secondary to complete mesenteric thrombosis. When TIPS and conventional surgical shunts are not an option to relieve pressure, esophageal devascularization can stop active hemorrhage and splenopneumopexy can potentially provide long-term venous decompression via the development of splenopulmonary collaterals.
Authors
Nicholas J. Skertich, MD
Rush University Medical Center
Department of Surgery, Division of Transplant Surgery
Charles Fredericks, MD
Rush University Medical Center
Department of Surgery, Division of Transplant Surgery
Lucas da Matta, MD
Rush University Medical Center
Department of Surgery, Division of Transplant Surgery
Erik Schadde, MD
Rush University Medical Center
Department of Surgery, Division of Transplant Surgery
Edie Y. Chan, MD
Rush University Medical Center
Department of Surgery, Division of Transplant Surgery
Martin Hertl, MD, PhD
Rush University Medical Center
Department of Surgery, Division of Transplant Surgery
Correspondence Author
Dr. Nicholas J. Skertich
Rush University Medical Center
Department of Surgery, Division of Transplant Surgery
1750 W. Harrison, Suite 785
Chicago, IL 60612
Phone: 708-606-3945
Email: Nicholas_J_Skertich@rush.edu
Meeting Presentation
Presented at Eastern Association for the Surgery of Trauma (EAST) 29th Annual Scientific Assembly, San Antonio, Texas, January, 2016
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- Garcia-Tsao G, Bosch J. Management of Varices and Variceal Hemorrhage in Cirrhosis. N Engl J Med. 2010;362(9):823–832.
- Gioia S, Nardelli S, Pasquale C, et al. Natural history of patients with non cirrhotic portal hypertension: Comparison with patients with compensated cirrhosis. Dig Liver Dis. 2018 Aug;50(8):839–844.
- Harnik IG, Brandt LJ. Mesenteric venous thrombosis. Vasc Med. 2010;15(5):407–418.
- Kumar S, Sarr MG, Kamath PS. Mesenteric venous thrombosis. N Engl J Med 2001;345(23):1683–1688.
- Sugiura M, Futagawa S. A new technique for treating esophageal varices. J Thorac Cardiovasc Surg. 1973;66(5):677–685.
- Bosch J, Berzigotti A, Garcia-Pagan JC, Abraldes JG. The management of portal hypertension: Rational basis, available treatments and future options. J Hepatol. 2008;48(SUPPL. 1).
- LaBreque D, Dite P, Fried M, Gangl A, Kan AG. Esophageal Varices. World Gastroenterol Organ Glob Guidel. 2014;(January):1–14.
- Tripathi D, Stanley AJ, Hayes PC, et al. UK guidelines on the management of variceal haemorrhage in cirrhotic patients. Gut. 2015;64(11):1680–1704.
- Ginsberg RJ, Waters PF, Zeldin RA, et al. A Modified Sugiura Procedure. Ann Thorac Surg. 1982;34(3):258–264.
- Akita, H, Sakoda K. Portopulmonary shunt by splenopneumopexy as a surgical treatment of Budd-Chiari syndrome. Surgery. 1980;87(1):85–94.