A) Normal biliary-type epithelium with small and basally located nuclei; B) focal area of high-grade dysplasia (biliary intraepithelial neoplasia) identified within cyst, characterized by epithelial cytologic dysplasia and architectural complexity
Discussion
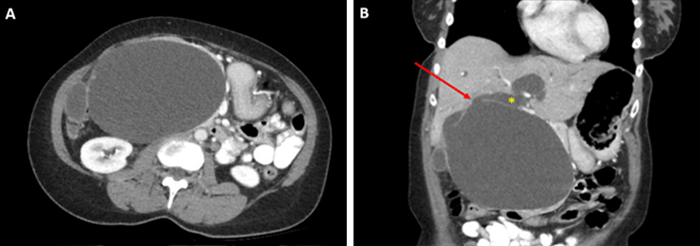
Choledochal cysts are rare congenital or acquired malformations of the intra- or extrahepatic biliary tree, typically diagnosed in childhood and more prevalent in East Asian populations. Fewer than 20 percent of cases are diagnosed after 20 years of age;1 however, incidence in asymptomatic adults is on the rise, principally due to incidental detection on cross-sectional imaging obtained for other reasons.2,3 When present, the predominant presenting symptom in adults is abdominal pain, often associated with cholangitis, pancreatitis, and cholelithiasis.4‒7 Abdominal masses and biliary obstruction are relatively uncommon presenting symptoms, particularly in the absence of overt malignancy.
The etiology of choledochal cysts is not completely understood, but various mechanisms for development have been proposed. Anomalous pancreaticobiliary duct union (APBDU), where the pancreatic and bile ducts join prior to duodenal insertion, is present in 30 to 70 percent of patients with choledochal cysts. It is theorized that increased exposure of the biliary epithelium to inflammation-inducing pancreatic enzymes may lead to cystic degeneration of the bile duct wall and cyst formation.8‒10 In one large study of nearly 3000 patients undergoing endoscopic retrograde cholangiopancreatography (ERCP), a choledochal cyst was found in 87 percent of patients with APBDU.11 Indirect evidence supporting this association includes elevation of cyst-fluid amylase levels and the development of significant common bile duct dilatation in mice undergoing iatrogenic APBDU.12,13 Increased intrabiliary pressure and sphincter of Oddi dysfunction have also been proposed as mechanisms leading to choledochal cyst development; however, given the chronicity and rarity of the disease, definitive causal relationships are difficult to establish.14,15
The most common diagnostic classification system introduced by Todani et al. establishes five types of choledochal cysts based on the primary anatomic location of biliary dilatation.16 Type I includes dilatation of the extrahepatic bile duct, with further subdivision into types IA, IB, and IC based on the location within the common bile duct and presence or absence of APBDU. A type II cyst is a saccular diverticulum of the common bile duct, distinct from the gallbladder. Type III cysts, also known as choledochoceles, are typically located in the duodenal wall. Their periampullary location can lead to pancreatitis, biliary obstruction, and, in rare cases, gastric outlet obstruction. This location also makes them more amenable to endoscopic intervention.17 Type IV cysts include both intra- and extrahepatic ductal dilatation or multiple areas of extrahepatic dilatation, while type V (Caroli's disease) involves multiple areas of intrahepatic dilatation.
The risk of malignant degeneration across all cyst types is estimated to be approximately 11 percent.5,7,18 Types I and IV are associated with the highest rates of malignancy, while type III cysts are rarely subject to malignant transformation.19 Overall, the risk of malignancy increases with age, further suggesting a link between chronic low-grade inflammation and cyst pathogenesis.20 Malignancy can occur even after surgical resection or cyst drainage. Patients who undergo drainage procedures (e.g., cystojejunostomy) are more than four times likelier to develop a subsequent biliary malignancy when compared to those undergoing complete cyst excision and hepaticojejunostomy, which is now the treatment of choice at most centers.19
Conclusion
In summary, this rare presentation of a choledochal cyst causing biliary obstruction without concomitant intraluminal malignancy underscores the chronicity and complexity of cyst development. The patient's biliary obstructive symptoms allowed for a targeted workup and rapid identification of her choledochal cyst, a diagnosis which can often be delayed due to vague presenting symptoms.21 Fortunately, her cyst was able to be treated before the development of more significant biliary complications such as cholangitis or a more life-threatening biliary malignancy. The utility of ongoing postoperative surveillance is unknown but likely low in patients undergoing complete cyst excision with biliary reconstruction.
Lessons Learned
The risk of malignancy increases with age, further suggesting a link between chronic low-grade inflammation and cyst pathogenesis. Additionally, patients who undergo drainage procedures are more likely to develop a subsequent biliary malignancy. This rare presentation of a choledochal cyst causing biliary obstruction without concomitant intraluminal malignancy underscores the chronicity and complexity of cyst development; the utility of ongoing postoperative surveillance is unknown but likely low in patients undergoing complete cyst excision with biliary reconstruction.
References
- Hewitt PM, Krige JE, Bornman PC, Terblanche J. Choledochal cysts in adults. Br J Surg. 1995;82(3):382-385. doi:10.1002/bjs.1800820333
- Dhupar R, Gulack B, Geller DA, Marsh JW, Gamblin TC. The changing presentation of choledochal cyst disease: an incidental diagnosis. HPB Surg. 2009;2009:103739. doi:10.1155/2009/103739
- Feng J-F, Chen W-Y, Chen D-F, Zhou S, Liu J. Choledochal cysts with malignancy in adult: a retrospective study with an experience of twenty-two years. Pak. J. Med. Sci. Q. 2011;27(1):6-10.
- Soares KC, Arnaoutakis DJ, Kamel I, et al. Choledochal cysts: presentation, clinical differentiation, and management. J Am Coll Surg. 2014;219(6):1167-1180. doi:10.1016/j.jamcollsurg.2014.04.023
- Edil BH, Cameron JL, Reddy S, et al. Choledochal cyst disease in children and adults: a 30-year single-institution experience. J Am Coll Surg. 2008;206(5):1000-1008. doi:10.1016/j.jamcollsurg.2007.12.045
- Wiseman K, Buczkowski AK, Chung SW, Francoeur J, Schaeffer D, Scudamore CH. Epidemiology, presentation, diagnosis, and outcomes of choledochal cysts in adults in an urban environment. Am J Surg. 2005;189(5):527-531. doi:10.1016/j.amjsurg.2005.01.025
- Moslim MA, Takahashi H, Seifarth FG, Walsh RM, Morris-Stiff G. Choledochal cyst disease in a western center: a 30-year experience. J Gastrointest Surg. 2016;20(8):1453-1463. doi:10.1007/s11605-016-3181-4
- Babbitt DP. [Congenital choledochal cysts: new etiological concept based on anomalous relationships of the common bile duct and pancreatic bulb]. Ann Radiol (Paris). 1969;12(3):231-240.
- Kimura K, Ohto M, Ono T, et al. Congenital cystic dilatation of the common bile duct: relationship to anomalous pancreaticobiliary ductal union. AJR Am J Roentgenol. 1977;128(4):571-577. doi:10.2214/ajr.128.4.571
- Jona JZ, Babbitt DP, Starshak RJ, LaPorta AJ, Glicklich M, Cohen RD. Anatomic observations and etiologic and surgical considerations in choledochal cyst. J Pediatr Surg. 1979;14(3):315-320. doi:10.1016/s0022-3468(79)80490-x
- Nagi B, Kochhar R, Bhasin D, Singh K. Endoscopic retrograde cholangiopancreatography in the evaluation of anomalous junction of the pancreaticobiliary duct and related disorders. Abdom Imaging. 2003;28(6):847-852. doi:10.1007/s00261-003-0031-0
- Miyano T, Suruga K, Suda K. "The choledocho-pancreatic long common channel disorders" in relation to the etiology of congenital biliary dilatation and other biliary tract disease. Ann Acad Med Singap. 1981;10(4):419-426.
- Yamashiro Y, Miyano T, Suruga K, et al. Experimental study of the pathogenesis of choledochal cyst and pancreatitis, with special reference to the role of bile acids and pancreatic enzymes in the anomalous choledocho-pancreatico ductal junction. J Pediatr Gastroenterol Nutr. 1984;3(5):721-727. doi:10.1097/00005176-198411000-00015
- Polido WT, Lorenzo JC, Tayag WY. Choledochal cyst in an adult: congenital or an acquired clinical entity?. WebmedCentral HEPATOBILIARY 2012;3(1):WMC002920
doi: 10.9754/journal.wmc.2012.002920
- Turowski C, Knisely AS, Davenport M. Role of pressure and pancreatic reflux in the aetiology of choledochal malformation. Br J Surg. 2011;98(9):1319-1326. doi:10.1002/bjs.7588
- Todani T, Watanabe Y, Narusue M, Tabuchi K, Okajima K. Congenital bile duct cysts: Classification, operative procedures, and review of thirty-seven cases including cancer arising from choledochal cyst. Am J Surg. 1977;134(2):263-269. doi:10.1016/0002-9610(77)90359-2
- Masetti R, Antinori A, Coppola R, et al. Choledochocele: changing trends in diagnosis and management. Surg Today. 1996;26(4):281-285. doi:10.1007/BF00311589
- Jan YY, Chen HM, Chen MF. Malignancy in choledochal cysts. Hepatogastroenterology. 2000;47(32):337-340.
- Ten Hove A, de Meijer VE, Hulscher JBF, de Kleine RHJ. Meta-analysis of risk of developing malignancy in congenital choledochal malformation. Br J Surg. 2018;105(5):482-490. doi:10.1002/bjs.10798
- Huang CS, Huang CC, Chen DF. Choledochal cysts: differences between pediatric and adult patients. J Gastrointest Surg. 2010;14(7):1105-1110. doi:10.1007/s11605-010-1209-8
- Stain SC, Guthrie CR, Yellin AE, Donovan AJ. Choledochal cyst in the adult. Ann Surg. 1995;222(2):128-133. doi:10.1097/00000658-199508000-00004
Authors
Clark NMa; Kolnik SMa; Gui Xb; Sham JGa
Author Affiliations
- Department of Surgery, University of Washington, Seattle, WA 98195
- Department of Pathology, University of Washington, Seattle, WA 98195
Corresponding Author
Jonathan G. Sham, MD
University of Washington
Box 356410
1959 Pacific Street NE
Seattle, WA 98195
Phone: (206) 685-5662
Email: jsham@uw.edu
Disclosure Statement
The authors have no conflicts of interest to disclose.
Funding/Support
The authors have no relevant financial relationships or in-kind support to disclose.
Received: July 19, 2020
Revision received: September 21, 2020
Accepted: November 1, 2020