Figure 3. Immunohistochemistry showing plasmablastic lymphoma of the adrenal gland. A: Sheets of pleomorphic and discohesive cells with hyperchromatic nuclei and prominent nucleoli [hematoxylin and eosin (H&E) staining, mag x 400]; B: Dysplastic cells were negative for B-cell marker CD20 [mag x 200]; C: Dysplastic cells were negative for synaptophysin, a neuroendocrine tumor marker [mag x 200]; D: Dysplastic cells were positive for the plasma cell marker CD138 [mag x 200]; E: Dysplastic cells were positive for the plasma cell marker MUM-1 [mag x 200]; F: Nuclei of the dysplastic cells were positive for Epstein Barr virus encoded RNA [mag x 200]; G: Dysplastic cells were positive for Ki-67 [mag x 200]; H: Positive markers show plasma cells expressing Lambda light chains [mag x 200]; I: Positive markers show Kappa light chain restriction, [mag x 200].
Discussion
Given that it has overlapping histological features of both myeloma and lymphoma, PBL poses significant diagnostic challenges amid its aggressive course and poor prognosis, with an estimated median overall survival ranging from 3 to 15 months among HIV-positive patients.5,6 Even among HIV-positive PBL patients receiving ART, such as the subject of our case study, the median overall survival of such patients has been found in retrospective case series to be under 12 months.7 Other post-ART era case series, however, have been more optimistic, with a recent retrospective analysis of 12 HIV-positive PBL patients on ART showing a one-year survival rate of 67 percent.8
While the pathogenesis of PBL remains unclear, both Myc gene rearrangements and an association with EBV infection have been posited to play an important role in allowing plasmablasts to escape apoptosis during maturation.6,9 Although expression of Ki-67 and EBV-related antigens in PBL, as seen in the case of our patient, have not yet been shown to hold prognostic value, the International Prognostic Index scoring (IPI) system for lymphomas (which includes age, performance status, LDH levels, number of extranodal sites, and clinical stage) has been found to prognosticate survival in PBL cases.6 No standard of care currently exists for PBL, with standard CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) chemotherapy regimens generally seen as inadequate therapy. While there is no evidence of improved survival with more intensive chemotherapeutic regimens,7 new therapeutic approaches under investigation include EPOCH and stem cell transplantation, as well as EBV- and Myc-directed medical therapies.6 New studies have also highlighted the capacity for DNA sequencing and the detection of circulating tumor DNA in patients’ blood to determine tumor cell origin and quantify micrometastatic burden in diffuse large B-cell lymphomas.10
Given its rarity and dismal prognosis, current knowledge of PBL remains grounded in case reports and case series with no prospective studies conducted to date. However, in a 2015 systematic review of 177 peer-reviewed case series and reports on PBL, of 590 patients, 63 percent were HIV-positive and 70 percent expressed EBV-encoded RNA.
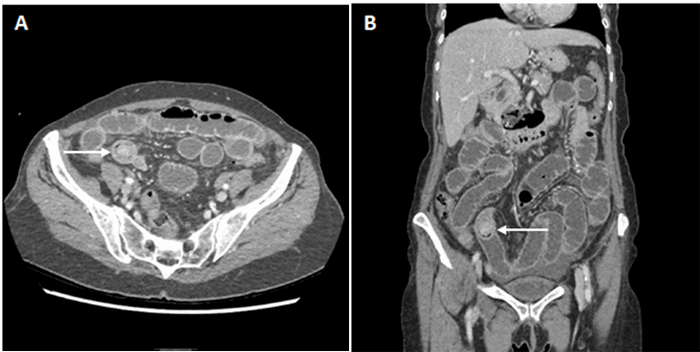
6Of the said HIV-positive patients, 95 percent presented extranodally with 48 percent involving the oral cavity and 40 percent with bone marrow involvement and B symptoms. There is only one other case report of adrenal PBL in the literature, found in a 55-year-old male AIDS patient on ART, who presented with fever and abdominal pain with a CD4 count of 72 at the time of diagnosis.
11To the best of our knowledge, this is the first case of adrenal PBL reported in a patient with well-controlled HIV on continuous ART. Resection of the patient’s incidental PBL provided an opportunity to diagnose the tumor at an early stage prior to any extra-adrenal involvement. In light of the limited literature base on PBL, it is essential to continue raising awareness of PBL and its diverse presentations in order to facilitate its accurate diagnosis.
Conclusion
Our case highlights a rare presentation of plasmablastic lymphoma in the adrenal gland of an HIV-positive patient with a normal CD4 count and an undetectable viral load.
Lessons Learned
Plasmablastic lymphoma is most common in HIV-positive patients and has a poor prognosis, even in post-HAART era patients adherent to antiretroviral medications. By demonstrating a case of PBL presenting as an incidental adrenal mass, our case further illustrates that PBL is highly heterogeneous in its presentations, leading its B symptoms to unexpectedly masquerade under the guise of pheochromocytoma and anxiety symptoms. Preoperative biopsy of adrenal mass, in the setting of no extra-adrenal malignancy, is not advisable as adrenal cortical carcinoma cannot be ruled out.
Authors
Xu KYa, Teng Ab, Mahabir Rc, Sharabi Ac, Fan Ac, Brody JDd, Inabnet WB 3rdb, Adimoolam De, Taye Ab
Correspondence Author
Aida Taye-Bellistri, MD
Department of Surgery, Mount Sinai St. Luke's Roosevelt
Icahn School of Medicine at Mount Sinai
1000 10th Avenue, Suite 2B10
New York, NY 10019
212-523-6051
aida.taye@mountsinai.org
Author Affiliations
- Department of Medical Education, Icahn School of Medicine at Mount Sinai, New York, NY
- Department of Surgery, Icahn School of Medicine at Mount Sinai, Mount Sinai Health System, New York, NY
- Department of Diagnostic Pathology and Laboratory Medicine, Mount Sinai Health System, New York, NY
- Division of Hematology-Oncology, Icahn School of Medicine at Mount Sinai, Mount Sinai Health System, New York, NY
- Division of Endocrinology, Diabetes, and Bone Disease, Icahn School of Medicine at Mount Sinai, Mount Sinai Health System, New York, NY
Author Contributions
KYX, AT, and ATB conceived and developed the arguments for the manuscript. KYX wrote the first draft. KYX, AT, ATB, RM, JB, WI, and DA participated in critical revisions of the manuscript for intellectual content. RM, AS, and WF analyzed and provided immunohistochemical content. All authors read and approved the final draft.
Disclosures/Competing Interests
We declare no competing interests.
References
- Carbone A. AIDS-related non-Hodgkin's lymphomas: from pathology and molecular pathogenesis to treatment. Hum Pathol.Apr 2002;33(4):392-404.
- Sarker AK, Im HJ, Paeng JC, et al. Plasmablastic lymphoma exclusively involving bones mimicking osteosarcoma in an immunocompetent patient: A case report. Medicine. Jul 2016;95(28):e4241.
- Zeiger MA, Thompson GB, Duh QY, et al. The American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons medical guidelines for the management of adrenal incidentalomas. Endocr Pract. Jul-Aug 2009;15 Suppl 1:1-20.
- Davison JM, Subramaniam RM, Surasi DS, Cooley T, Mercier G, Peller PJ. FDG PET/CT in patients with HIV.Am J Roentgenol. Aug 2011;197(2):284-294.
- Castillo J, Pantanowitz L, Dezube BJ. HIV-associated plasmablastic lymphoma: lessons learned from 112 published cases. Am J Hematol. Oct 2008;83(10):804-809.
- Castillo JJ, Bibas M, Miranda RN. The biology and treatment of plasmablastic lymphoma. Blood. Apr 09 2015;125(15):2323-2330.
- Castillo JJ, Furman M, Beltran BE, et al. Human immunodeficiency virus-associated plasmablastic lymphoma: poor prognosis in the era of highly active antiretroviral therapy. Cancer. Nov 01 2012;118(21):5270-5277.
- Noy A, Lensing SY, Moore PC, et al. Plasmablastic lymphoma is treatable in the HAART era. A 10 year retrospective by the AIDS Malignancy Consortium. Leuk Lymphoma. Jul 2016;57(7):1731-1734.
- Klapproth K, Wirth T. Advances in the understanding of MYC-induced lymphomagenesis. Br J Haematol. May 2010;149(4):484-497.
- Scherer F, Kurtz DM, Newman AM, et al. Distinct biological subtypes and patterns of genome evolution in lymphoma revealed by circulating tumor DNA. Sci Transl Med. Nov 09 2016;8(364):364ra155.
- Bishnu S, Banerjee S, Bandyopadhyay D, Samui S, Bhattacharya S, Bose D. Plasmablastic lymphoma in HIV patients: Experience at a tertiary care hospital in eastern India. Indian J Cancer. Oct-Dec 2015;52(4):563-567.



![Figure 3. Immunohistochemistry showing plasmablastic lymphoma of the adrenal gland. A: Sheets of pleomorphic and discohesive cells with hyperchromatic nuclei and prominent nucleoli [hematoxylin and eosin (H&E) staining, mag x 400]; B: Dysplastic cells were negative for B-cell marker CD20 [mag x 200]; C: Dysplastic cells were negative for synaptophysin, a neuroendocrine tumor marker [mag x 200]; D: Dysplastic cells were positive for the plasma cell marker CD138 [mag x 200]; E: Dysplastic cells were positive for the plasma cell marker MUM-1 [mag x 200]; F: Nuclei of the dysplastic cells were positive for Epstein Barr virus encoded RNA [mag x 200]; G: Dysplastic cells were positive for Ki-67 [mag x 200]; H: Positive markers show plasma cells expressing Lambda light chains [mag x 200]; I: Positive markers show Kappa light chain restriction, [mag x 200].](/media/j2hjamqr/cr4_fig3.png?rnd=132950735834270000)