Abstract
Background
Abdominal mesh for herniorrhaphy has been associated with various complications. One rare complication involves mesh migration or erosion into an abdominal or pelvic organ. We describe an interesting case of complete mesh migration into the small bowel in a 65-year-old male three years after laparoscopic parastomal hernia repair.
Summary
The patient complained of one year of weekly episodes of self-resolving obstructive symptoms with decreased ostomy output. CT imaging showed a large parastomal hernia recurrence and an intraluminal density of unclear significance within the small bowel. Exploratory laparotomy demonstrated a large and mobile bezoar within the ileum which was extracted via an enterotomy and found to be the previously placed abdominal mesh; there was no identifiable fistula tract.
Conclusion
This case demonstrates that mesh migration into the bowel should be considered in patients with chronic obstructive symptoms following hernia repair, especially if there is CT evidence of an intraluminal bowel density.
Key Words
mesh migration; parastomal hernia; synthetic mesh; hernia recurrence; small bowel
Case Description
Abdominal hernia repairs are among the most common surgical procedures performed by general surgeons.1,2 The advent of synthetic mesh to repair abdominal hernias has drastically reduced hernia recurrence rates.3‒5 However, the use of synthetic mesh has been associated with numerous complications6‒8 and is the source of much research.9‒12 One rare but more recently described complication includes mesh migration or erosion into an abdominal or pelvic organ. Migration refers to whole mesh displacement into an organ, while erosion represents the partial perforation of a mesh into an organ with a portion remaining outside.13 In particular, inguinal mesh erosion into the sigmoid or bladder following inguinal hernia repair has been frequently reported,14,15 and reports of erosion into the large bowel and small bowel following ventral and inguinal repairs have also been described.13 We describe an interesting case of complete mesh migration into the small bowel in a 65-year-old male three years after undergoing a laparoscopic parastomal hernia repair.
A 59-year-old obese male with a history of hypertension and a 25-pack-year smoking history underwent a laparoscopic abdominoperineal resection with the placement of a left-sided end colostomy for rectal adenocarcinoma. Subsequently, he underwent an elective laparoscopic parastomal hernia repair with mesh due to obstructive symptoms at the age of 62 (body mass index [BMI]=40). Prior to his hernia repair, the patient had weekly obstructive episodes characterized by hernia pain, nausea, vomiting, and decreased ostomy output. The patient was advised to undergo a weight loss plan and quit smoking and was reevaluated two months later with continued obstructive episodes and only a net weight loss of one pound. It was believed at that time that due to the frequency and severity of his obstructive episodes, hernia repair could not be delayed for the patient to have significant weight loss. Thus, he underwent a scheduled hernia repair. His hernia defect was approximately 5 × 7 cm. The repair included a 15 × 19 cm Gore® DualMesh® placed intraperitoneal using a Sugarbaker technique with four corner transfascial permanent sutures and additional tacks in each quadrant. Unfortunately, eight months later, he developed a recurrence of his parastomal hernia with a non-dilated loop of transverse colon within the hernia sac superior to his stoma. Immediate repair was not undertaken given a paucity of symptoms, and weight loss was encouraged. Of note, the patient had quit smoking in the weeks leading up to his primary hernia repair; however, he returned to smoking in the weeks after his surgery.
At 65 years old, he presented complaining of greater than one year of weekly episodes of abdominal pain, decreased colostomy output, nausea, and vomiting, eventually followed by high ostomy output and symptom resolution. He was morbidly obese (BMI=36) on physical exam with a large incarcerated nonreducible left parastomal hernia extending 12 cm cephalad with palpable bowel.
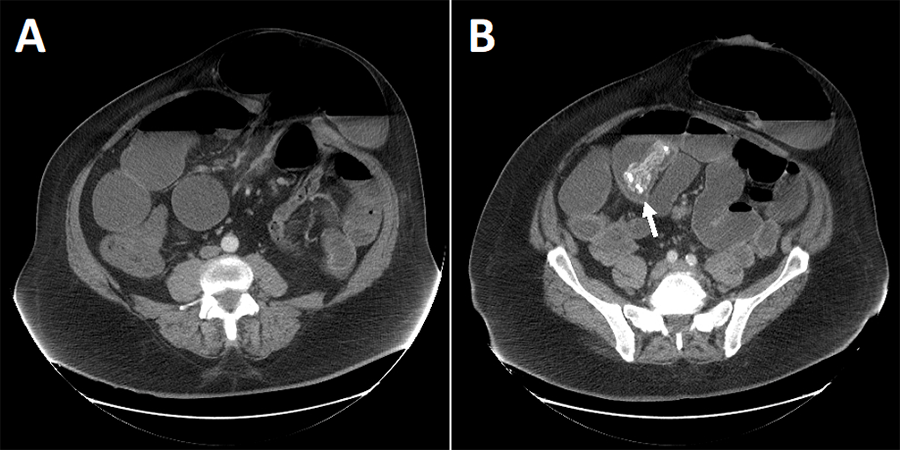
Computed tomography (CT) imagining demonstrated dilated bowel up to 8 cm in diameter with air-fluid levels proximal to and distal to the parastomal hernia consistent with an obstructive process (Figure 1A). Additionally, there was noted to be an intraluminal density of unclear significance within the small bowel, presumed to be fecalized small bowel contents that did not appear to be a source of obstruction (Figure 1B). Compared to two prior annual cancer follow-up CT scans performed one and two years before this study, the size of the parastomal hernia was similar. However, the small bowel proximal and distal to the hernia were notably more dilated in the study shown here. Additionally, prior CT scans did not demonstrate any intraluminal density and showed the mesh at the peritoneal wall with parastomal hernia recurrence.
Figure 1. Computed Tomography (CT) of Abdomen and Pelvis. Published With Permission
Contrast demonstrates A) parastomal hernia sac and loops of dilated bowel and B) intraluminal density within small bowel of unclear significance.
Upon reevaluation, the patient was scheduled for an elective open repair of his hernia with the removal of mesh and resiting his colostomy with possible partial colectomy. Of note, the patient had an unremarkable colonoscopy for cancer screening purposes performed through his colostomy 13 months prior to his proposed parastomal hernia recurrence repair.
The patient was taken to the operating room 37 months after his initial hernia repair. After exposing the hernia defect, the only content within the hernia was the descending colon approaching the colostomy adhered to the hernia sac. The abdominal mesh was unable to be visualized along the abdominal fascia. A large palpable bezoar approximately 2.5 cm in diameter and 8 cm in length was noted within the ileum, and the small bowel was dilated proximally and distally. Due to adherent loops of terminal ileum within the pelvis, the bezoar was milked back to the dilated mid jejunum, and an enterotomy was created for extraction. The foreign body was then unfurled and noted to be the Gore® mesh intact with all corner tacking sutures and numerous ProTack™ tacks (Figure 2).
Figure 2. Explanted Abdominal Mesh With All 4-Corner Tacking Sutures in Place. Published With Permission
There was no identifiable fistula tract between any portion of the colon or the small intestine and the small bowel was not adherent anywhere near the colostomy site. Due to adhesive disease of the distal colon within the hernia sac and the need to resite the colostomy to the contralateral location, a partial left colectomy was performed, and the colostomy was resited to the right side of the abdomen. The plastic surgery team then assisted with the closure of the abdominal cavity. First, the left-sided colostomy defect was repaired. Internally, the rectus abdominis muscle was repaired vertically, and the internal rectus fascia was then repaired with #1 Maxon™ monofilament absorbable sutures. A patch of the preperitoneal flap was then placed over this repair internally. Externally, the rectus was repaired with mattress sutures, and then a transverse repair of the external fascial defect, which was 7 cm in length, was repaired with #0 Maxon™ sutures. Next, the abdominal wall reconstruction was completed with an anterior component separation with left and right rectus abdominis myocutaneous perforator sparing advancement flaps.
The patient recovered well postoperatively. After five days, he regained bowel function and was quickly able to tolerate enteral feeding with well-controlled postoperative pain and was then discharged.
Seventeen months since his procedure, he feels remarkably better. Unfortunately, he has had a recurrence of abdominal hernias with one near his right-sided stoma and one in the left abdomen where his previous colostomy was sited. However, he states that he no longer has any nausea, vomiting, or abdominal pain and can eat well without constipation or obstructive symptoms. He does not wish to have any hernia repair currently.