IV Fluid Composition Studies
SPLIT Trial
The SPLIT (0.9 percent Saline vs Plasma-Lyte 148 (PL-148) for ICU fluid Therapy) trial10 was a double-blind RCT that randomized adult ICU patients (n=2,278) across four ICUs in New Zealand to receive PlasmaLyte versus 0.9 percent saline. There was a median two liters IV fluid administered in each arm. No difference in 90-day acute kidney injury, renal replacement therapy, or in-hospital mortality was identified.
SALT and SALT-ED Trials
The SALT (Saline against Lactated Ringer’s or Plasma-Lyte) trial11 was a pilot study that compared balanced crystalloids versus saline in 974 adult patients in a single medical ICU, most admitted from the emergency department with sepsis. No difference in mortality was noted, but there was a lower rate of Major Adverse Kidney Event at 30 days (MAKE30, death from any cause, new renal-replacement therapy, or persistent renal dysfunction) with balanced crystalloids in sepsis patients and those who received larger volumes of IV fluids. The SALT-ED (Saline against Lactated Ringer’s or Plasma-Lyte in the Emergency Department) trial12 randomized 13,347 emergency department patients at a single center who required IV fluid resuscitation to balanced crystalloids or saline. No difference in the primary outcome of hospital-free days was noted, but balanced crystalloids had a significantly lower rate of MAKE30, and this was greatest among the subgroup of patients who had an elevated plasma creatinine on presentation and in patients with hyperchloremia. Limitations of these two trials include the open label design and initiation of renal replacement therapy (RRT) was determined by the treating clinicians.
SMART Trial
The SMART (Isotonic Solutions and Major Adverse Renal Events) trial13 was a non-blinded RCT that randomized critically ill adults (n=15,802) across five ICUs in a single U.S. academic center (Vanderbilt). IV fluids were alternated monthly between balanced crystalloid (lactated Ringer’s solution or PlasmaLyte A) and 0.9 percent saline, beginning in the emergency department and continued in the ICU. Balanced crystalloids resulted in a lower rate of the composite outcome MAKE30 compared to saline (14.3 percent versus 15.4 percent, OR 0.91, p = 0.04). Interestingly, the difference in the composite outcome (MAKE30) was driven by death (OR 0.9; 95 percent CI 0.80 – 1.01) and RRT (OR 0.84; 95 percent CI 0.68-1.02) and not by changes in creatinine. No difference in need for dialysis (2.9 percent saline versus 2.5 percent) or persistent renal dysfunction (6.6 percent versus 6.4 percent) was noted. In the surgical patient planned subgroup analysis, no difference in MAKE30 was identified (11.4 percent versus 12.2 percent).
Subgroup analysis confirmed that normal saline was associated with increased mortality among patients with sepsis (29.4 percent versus 25.2 percent; p=0.02) or chronic dialysis (18.4 percent versus 12.2 percent; p=0.01). Furthermore, the difference in outcomes between balanced crystalloids and saline was greater for patients with sepsis and patients who received larger volumes of crystalloid. A post-hoc analysis of sepsis medical ICU patients (n=1,641) reported higher 30-day in-hospital mortality in the saline group (31.2 percent versus 26.3percent, OR 0.74, CI 0.59 – 0.93, p = 0.001), with a number needed to treat with balanced crystalloids to prevent one death of 20.14
BaSICS Trial
The BaSICS (Balanced Solution versus Saline in Intensive Care Study) trial15 was conducted in hopes to settle the divergent results of the SPLIT and SMART trials discussed above. BaSICS was a multicenter double-blind RCT that randomized adult ICU patients (n=11,052) on the first ICU day in 75 ICUs in Brazil to balanced crystalloid (PlasmaLyte 148) versus 0.9 percent saline for all ICU fluid administration (boluses, maintenance, and carriers) with a primary outcome measure of 90-day all-cause mortality. There was no significant different in 90-day mortality (26.4 percent PlasmaLyte versus 27.2 percent 0.9 percent saline; p=0.47). Subgroup analysis showed no statistically significant difference in acute kidney injury (27 percent versus 27 percent) or need for dialysis (1 percent versus 1 percent). Subgroup analysis also confirmed higher mortality in patients with traumatic brain injury who received balanced crystalloids (31.3 percent PlasmaLyte 148 versus 21.1 percent normal saline; hazard ratio 1.48, 95 percent CI 1.03 – 2.12, p=0.02).
Unlike the SMART trial, the BaSICS trial failed to demonstrate decreased mortality, AKI, or need for RRT with the use of balanced crystalloid. Significant limitations of the BaSICS trial are the low volume of IV fluid administered (median 1.5 L on the first ICU day), low severity of illness and approximately 80 percent compliance with study fluid administration (compared to 95 percent in the SMART trial). Additionally, 48 percent of the cohort were ICU admissions after elective surgery, with low severity of illness and low risk of acute kidney injury. In contrast, the SMART trial enrolled mostly unplanned ICU admissions with higher severity of illness and fewer elective surgical patients. The BaSICS trial, however, had a much higher in-hospital mortality rate (23 percent) compared to the SMART trial (11 percent).
Rate of IV Fluid Administration
The BaSICS trial16 also studied the rate of bolus IV crystalloid infusion (333 versus 999 ml/hr), and there was no significant difference in 90-day mortality between the slower infusion group versus the rapid infusion cohort (26.6 percent versus 27 percent; p=0.46).
Systematic Reviews
A systematic review of intervention trials of critically ill adult patients (n=26,351 in 58 trials) who required fluid resuscitation separately examined outcomes in surgical, trauma, traumatic brain injury, and sepsis patient cohorts. For surgery and sepsis patients, balanced crystalloids and albumin were associated with increased survival, decreased red blood cell transfusion volume, and decreased acute kidney injury. In patients with traumatic brain injury, saline was associated with decreased mortality compared to albumin and balanced crystalloids.17 This systematic review, however, did not include the recently published BaSICS trial.
Future Studies
Additional data will soon be available from the multicenter Plasma-Lyte 148 versUs Saline Study (PLUS) from the Australian and New Zealand Intensive Care Society Clinical Trials Group (ANZICS), as it has recently completed enrollment (n=5,037). Adult ICU patients receiving an IV fluid bolus for evidence of hypovolemia were randomized to receive either PlasmaLyte 148 or 0.9 percent normal saline for all crystalloid therapy and all resuscitation episodes for up to 90 days after randomization. The primary outcome measure of the PLUS multicenter blinded RCT is all-cause 90-day mortality, so it will provide comparable data to the BaSICS trial.18-20 Importantly, the PLUS study excluded patients with traumatic brain injury and those at risk for developing cerebral edema based on potential harm with balanced crystalloids identified in the other clinical trials. Another study, BEST-FLUIDS (Better Evidence for Selecting Transplant Fluids), is a multicenter, double-blind RCT comparing PlasmaLyte 148 versus 0.9 percent saline on the incidence of delayed graft function in deceased donor kidney transplant recipients (n=800).21
Conclusion and Clinical Recommendations
Almost all patients in the ICU receive IV fluids, but what fluids are best based on the studies to date? Given the divergent outcomes of the two largest trials (SMART and BaSICS) with different primary outcome measures (MAKE30 versus 90-day mortality) and enrollment of different patient populations (higher severity of illness, less elective surgical patients in SMART versus lower severity of illness and more elective surgical patients in BaSICS), how should these data impact our clinical practice?
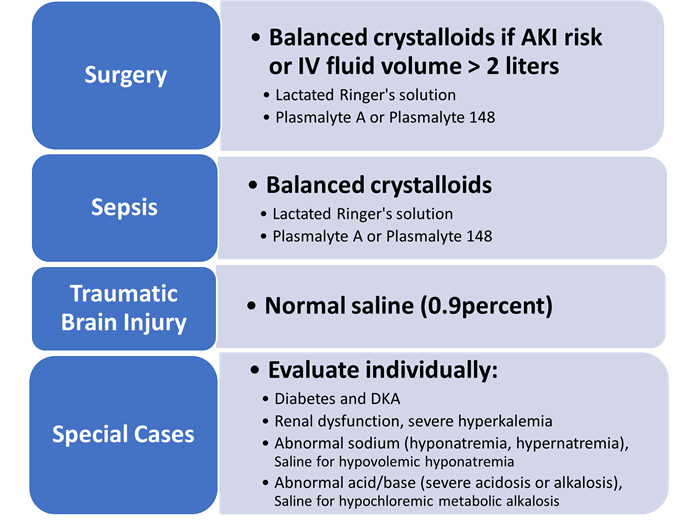
First, all trials to date and the latest systematic review have confirmed a clear significant benefit to 0.9 percent saline in traumatic brain injury (TBI) patients (Figure 1). Second, if the patient is likely to receive two liters of IV fluid or less and has no risk factors for acute kidney injury (like many of our elective surgical patients), then either crystalloid (balanced or saline) is likely safe.
Figure 1. Optimal Resuscitation IV Fluids in Adult Critical Care