Abstract
Background
There are fewer than 70 reported cases characterizing pancreatitis, panniculitis, and polyarthritis (PPP) syndrome—a rare triad consisting of polyarthritis with intraosseous fat necrosis, lobular panniculitis, and acute or chronic pancreatitis (or pancreatic malignancy). Surgery to address the underlying pancreatic pathology may play a crucial role in treatment but is seldom described in the literature.
Summary
We describe a case of a 60-year-old male with a history of chronic pancreatitis who presented with several weeks of pain and swelling of multiple joints, soft tissue nodules, and serum lipase over 11,000 U/L. Extensive rheumatologic workup was negative, and nodule biopsy findings were suggestive of PPP syndrome. He subsequently underwent pancreaticoduodenectomy to address a suspected pancreatomesenteric fistula. The fistula was confirmed intraoperatively between a necrotic cavity in the uncinate process, and the superior mesenteric vein (SMV), and the SMV defect was repaired. Postoperatively, the patient had a marked reduction in amylase and lipase, significantly improving systemic symptoms.
Conclusion
Fistulization between the pancreas and the mesenteric venous system in PPP syndrome results in an uncontrolled release of pancreatic enzymes into the systemic circulation. This can lead to enzymatic degradation of fat throughout the body, which manifests as panniculitis with necrotizing neutrophilic inflammation and polyarthritis with intraosseous fat necrosis. Pancreatic and fistula resection can lead to a pronounced reduction in serum pancreatic enzyme levels with improvement in extrapancreatic manifestations of the disease. This case brings additional awareness to a rare diagnosis and the importance of surgical management in select patients.
Key Words
pancreatitis; panniculitis; polyarthritis; PPP syndrome; pancreaticoduodenectomy
Abbreviations
PPP syndrome: pancreatitis, panniculitis, and polyarthritis syndrome
SMV: superior mesenteric vein
MCP: metacarpophalangeal
CRP: C-reactive protein
ESR: erythrocyte sedimentation rate
WBC: white blood cell
ANA: antinuclear antibody
ANCA: antineutrophil cytoplasmic antibody
MRI: magnetic resonance imaging
CT: computerized tomography
Case Description
The patient is a 60-year-old male diagnosed with pancreatitis in 2017, with two subsequent hospitalizations and the development of a pancreatic pseudocyst. He presented again in January of 2021 with intermittent mild abdominal pain, severe right ankle pain, and swelling that had progressed over three to four weeks. He was noted to have a leukocytosis and elevated serum lipase over 11,000 U/L. His right ankle was aspirated with no growth on cultures, no crystals seen, and no improvement with nonsteroidal anti-inflammatory drugs, systemic corticosteroids, or antibiotics. The patient was transferred to the University of Washington Medical Center for further workup and treatment.
He had an additional past medical history notable for atrial fibrillation and pulmonary embolism but took no medications. At the time of presentation, he was experiencing homelessness and reported intermittent alcohol use but no other drug use.
On examination, his abdomen was nontender to palpation. He was noted to have multiple joints that were erythematous, swollen, and tender, including his right ankle, left wrist, and right third metacarpophalangeal (MCP) joint (Figure 1). Nodules identified on his left wrist, right medial malleolus, and left plantar surface were in various stages of healing and draining purulent, non-odorous, brown discharge. A dermatology consult team found these nodules to be compatible with panniculitis.
Figure 1. Photograph Demonstrating Erythematous and Swollen Left Wrist and Right Third MCP Joint Noted on Physical Exam. Published with Permission
Laboratory studies revealed serum lipase 11,475 U/L; amylase 1645 U/L; C-reactive protein (CRP) 84.9 mg/L; erythrocyte sedimentation rate (ESR) 22 mm/hr; white blood cell (WBC) count 21 × 109/L; negative antinuclear antibody (ANA), antineutrophil cytoplasmic antibody (ANCA), myeloperoxidase, and proteinase 3; and uric acid 4.1 mg/dL.
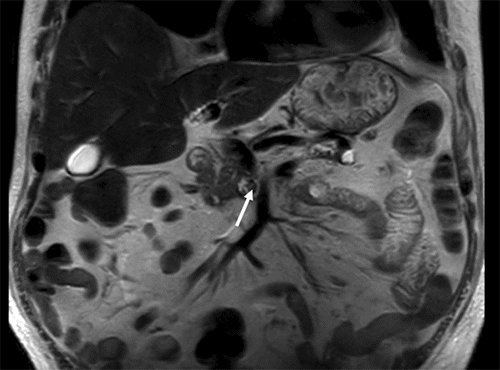
Magnetic resonance imaging (MRI) of the right lower extremity showed findings consistent with noninfectious osteomyelitis—diffuse soft tissue edema around the right ankle with effusion, fluid in the posterior tibialis tendon sheath with synovial and muscle enhancement, and osseous hyperenhancement of the mid-tibial shaft with periosteal edema (Figure 2). Computerized tomography (CT) scan of the abdomen and pelvis with IV contrast was notable for soft tissue stranding around the pancreatic head, a large pancreatolith in the body of the pancreas, and a non-occlusive superior mesenteric vein (SMV) thrombus (Figure 3). MRI of the abdomen demonstrated pancreatic ductal dilation and a focal area of pancreatic necrosis in the uncinate directly adjacent to the SMV (Figure 4).
Figure 2. CT Scans. Published with Permission
A.
Upper endoscopy with endoscopic ultrasound was performed to evaluate for malignancy—no pancreatic mass was identified. Given the draining nodules on physical exam were suggestive of fat necrosis and an extensive rheumatologic workup of the patient's polyarthritis was negative, a skin biopsy of the left wrist nodule was performed. This demonstrated dense dermal and subcutaneous necrotizing neutrophilic inflammation consistent with pancreatic panniculitis (Figure 5).1 Wound culture from the nodules was negative for pathogenic organisms.
Figure 5. Representative Histopathological Images at (A) 20x, (B) 100x, and (C) 200x Magnification of Left Wrist Punch Biopsy. Published with Permission
A.
Due to clinical, radiologic, and pathologic findings consistent with PPP syndrome, the patient underwent pancreaticoduodenectomy (Whipple procedure). Severe inflammation in the lesser sac and a fistula between a necrotic cavity in the uncinate process to the SMV was noted intraoperatively (Figure 6). The pancreatic resection, which included resection of the fistula, was extended through the neck to include the pancreatic duct stone that could not be retrieved and the SMV defect was repaired primarily. The remainder of the resection and reconstruction were uneventful.
Figure 6. Intraoperative Photograph After Division of Pancreas and Dissection of Superior Mesenteric Vein (SMV), Splenic Vein (SV), and Portal Vein (PV). Published with Permission
The patient recovered from surgery without complications. His polyarticular pain and dermatologic manifestations rapidly improved. Notably, serum amylase and lipase levels quickly reduced to 23 U/L and 3 U/L, respectively, and the patient was discharged from the hospital after a normal postoperative course. The pathology from his surgical specimen was notable for no malignancy and findings of chronic pancreatitis, including pseudocyst and intra- and peri-pancreatic fat necrosis. Additionally, an ectatic duct with necrosis and fistula formation in the region of the SMV margin was noted (Figure 7).
Figure 7. Photograph of Surgical Specimen. Published with Permission
Discussion
Pancreatitis, panniculitis, and polyarthritis (PPP) syndrome is a rare triad encountered in patients with benign and malignant pancreatic disease. With fewer than 70 reported cases in the literature, this uncommon constellation of clinical findings consists of polyarthritis with intraosseous fat necrosis, lobular panniculitis with necrotizing neutrophilic inflammation, and acute or chronic pancreatitis, or pancreatic malignancy.1-3 While recent literature has characterized PPP syndrome and challenges with diagnosis and management, a description of surgery's role in treating the disease is lacking.
PPP syndrome is an exceedingly rare presentation of potentially debilitating extrapancreatic manifestations of primary pancreatic pathology.4 Demographically, PPP syndrome most commonly affects middle-aged men with a history of alcohol use, as in this case.1,2 Up to 60% of cases have been associated with acute pancreatitis, approximately 40% with chronic pancreatitis, and 12-47% with pancreatic malignancy.1-3,5-7 Up to two-thirds of patients have mild and non-specific abdominal symptoms, contributing to clinically significant delays in diagnosis.2,8,9 Laboratory values typically include elevated pancreatic enzymes and acute phase reactants.1
Additional clinical features include arthritis, most commonly polyarthritis but occasionally mono- or oligoarthritis. There is a predilection for large joint involvement, including, in descending order, ankles, knees, MCP joints, and wrists.1,2 Arthritis typically develops three to six weeks after the peak of the pancreatitis episode and may involve rapid progression to joint damage and pathologic fractures.2,10-12 MRI is the most sensitive imaging modality for detecting early fatty marrow changes, foreshadowing the classic radiographic finding of intraosseous fat necrosis.2,13,14 Other frequent radiographic findings include osteolytic lesions, moth-eaten bone destruction, loss of joint space, and periostitis.2
The lobular panniculitis characteristic of PPP syndrome presents most commonly as erythematous nodules on the lower extremities, which can be either tender or painless. It is thus often confused with erythema nodosum initially; however, migration of the nodules to the abdomen or arms is a distinguishing factor.1,2 The characteristic histology—necrotizing neutrophilic pan-lobular inflammation with or without saponified "ghost-like" adipocytes in areas of fat necrosis—is distinct from the typically septal panniculitis of erythema nodosum.15,16 Nodules often evolve into necrotic abscesses with ulceration and drainage of brown, purulent material,17 and infection must be ruled out.
While an underlying cause is not clear in all cases, the proposed mechanism for joint and skin involvement is the release of lipase and other pancreatic enzymes directly into systemic circulation via a fistulous connection with the pancreas. This induces lipolysis, fatty acid accumulation, and secondary inflammation in the synovium, bone marrow, and soft tissues.1,2,18-22 In support of this pathway, high lipase levels are found in the synovial fluid of patients with PPP syndrome and correlate with the severity of extrapancreatic fat necrosis.8,11
Treatment is directed at the underlying pancreatic disease, which in the case of acute and chronic pancreatitis has classically involved supportive medical treatment. Nonsteroidal anti-inflammatory drugs and corticosteroids are often used for symptomatic management of polyarthritis and panniculitis, however, less than one in five patients exhibit a meaningful response to these therapies alone without control of the pancreatic process.1
While treatment of pancreatitis relies primarily on medical therapies, the pathophysiology of PPP syndrome is distinctive and may warrant a more directed surgical approach to prevent ongoing leakage of damaging pancreatic enzymes into systemic circulation.
Despite this important role for operative intervention in successful treatment, we found only three articles that describe pancreatic surgery for patients with PPP syndrome: necrosectomy via sinus endoscopy after failed step-up approach in a patient with necrotizing pancreatitis,23 pancreatic head resection and SMV reconstruction in a patient with a history of necrotizing pancreatitis complicated by pseudocyst and fistula to the SMV,11 and spleen-preserving total pancreatectomy in a patient with chronic pancreatitis found to have significant peripancreatic necrosis.6 Of note, all three patients had complete remission of joint pain and panniculitis following recovery from surgery.
In congruence with the proposed pathophysiology of PPP syndrome, our patient was found to have a necrotic cavity in the uncinate with fistulization to the SMV. The SMV defect was repaired, and the pancreaticoduodenectomy specimen included the uncinate, thus interrupting the passage of degradative enzymes into the bloodstream and, subsequently, the synovium, bone marrow, and soft tissues. Like other patients with PPP syndrome who have undergone surgery, our patient's serum amylase and lipase rapidly normalized, and extrapancreatic manifestations notably improved postoperatively.
Overall, the prognosis for PPP syndrome is mixed. Up to 80% of patients have a poor response to symptomatic and medical treatment, with evolution to chronicity and enduring functional impairment. Moreover, approximately 1 in 4 patients die from pancreatitis-related complications at a median duration of eight weeks.1,2 However, as demonstrated by this patient and the three above-described cases involving operative resection, PPP syndrome is potentially curable when a surgically correctable anatomic abnormality such as a pancreatomesenteric fistula can be identified and treated.6,11,23
Conclusion
Fistulization to the mesenteric venous system in PPP syndrome may be the cause of the ongoing release of pancreatic enzymes into systemic circulation, thus inciting and perpetuating the hallmark clinical signs of polyarthritis and panniculitis. Pancreatic and fistula resection can lead to a rapid reduction in serum pancreatic enzyme levels with improvement in or resolution of extrapancreatic manifestations. This case brings additional awareness to a rare diagnosis and the importance of surgical management in select patients.
Lessons Learned
A pancreatomesenteric fistula caused by pancreatitis or malignancy is the likely etiology of the persistently elevated serum pancreatic enzyme levels and the resultant dermatologic and rheumatologic manifestations characteristic of PPP syndrome. Surgical management may be required to address this underlying pathology and offer the best chance for symptom resolution.
LKD, KMS, and JGS contributed to the conception of the work. All authors contributed to the interpretation of the patient information, including laboratory values, radiologic images, operative findings, and histopathology findings. All authors drafted the article and/or substantively revised it. All authors read and approved the final version to be submitted.
Authors
Dickerson LKa; Sullivan KMa; Chew FSb; Swanson PEc; Long THc; Wells Dd; Pillarisetty VGa; Sham JGa
Author Affiliations
- Department of Surgery, University of Washington, Seattle, WA 98195
- Department of Radiology, University of Washington, Seattle, WA 98195
- Department of Pathology, University of Washington, Seattle, WA 98195
- Department of Medicine, Dermatology Division, University of Washington, Seattle, WA 98195
Corresponding Author
Jonathan G. Sham, MD
Department of Surgery
University of Washington
1959 NE Pacific Street
Seattle, WA 98195
Email: jsham@uw.edu
Disclosure Statement
The authors have no conflicts of interest to disclose.
Funding/Support
The authors have no relevant financial relationships or in-kind support to disclose.
Received: June 17, 2021
Accepted: August 18, 2021
References
- Betrains A, Rosseels W, Van Mieghem E, Vanderschueren S, Nijs J. Clinical characteristics, treatment, and outcome of pancreatitis, panniculitis, and polyarthritis syndrome: a case-based review. Clin Rheumatol. 2021;40(4):1625-1633. doi:10.1007/s10067-020-05333-8
- Narváez J, Bianchi MM, Santo P, et al. Pancreatitis, panniculitis, and polyarthritis. Semin Arthritis Rheum. 2010;39(5):417-423. doi:10.1016/j.semarthrit.2008.10.001
- Tannenbaum H, Anderson LG, Schur PH. Association of polyarthritis, subcutaneous nodules, and pancreatic disease. J Rheumatol. 1975;2(1):15-20.
- Chattopadhyay A, Mittal S, Sharma A, Jain S. Pancreatitis, Panniculitis, and Polyarthritis. J Clin Rheumatol. 2020;26(5):e90. doi:10.1097/RHU.0000000000000914
- Radin DR, Colletti PM, Forrester DM, Tang WW. Pancreatic acinar cell carcinoma with subcutaneous and intraosseous fat necrosis. Radiology. 1986;158(1):67-68. doi:10.1148/radiology.158.1.3940400
- Ferri V, Ielpo B, Duran H, et al. Pancreatic disease, panniculitis, polyarthrtitis syndrome successfully treated with total pancreatectomy: Case report and literature review. Int J Surg Case Rep. 2016;28:223-226. doi:10.1016/j.ijscr.2016.09.019
- Arbeláez-Cortés A, Vanegas-García AL, Restrepo-Escobar M, Correa-Londoño LA, González-Naranjo LA. Polyarthritis and pancreatic panniculitis associated with pancreatic carcinoma: review of the literature. J Clin Rheumatol. 2014;20(8):433-436. doi:10.1097/RHU.0000000000000181
- Boswell SH, Baylin GJ. Metastatic fat necrosis and lytic bone lesions in a patient with painless acute pancreatitis. Radiology. 1973;106(1):85-86. doi:10.1148/106.1.85
- Simkin PA, Brunzell JD, Wisner D, Fiechtner JJ, Carlin JS, Willkens RF. Free fatty acids in the pancreatitic arthritis syndrome. Arthritis Rheum. 1983;26(2):127-132. doi:10.1002/art.1780260202
- Mourad FH, Hannoush HM, Bahlawan M, Uthman I, Uthman S. Panniculitis and arthritis as the presenting manifestation of chronic pancreatitis. J Clin Gastroenterol. 2001;32(3):259-261. doi:10.1097/00004836-200103000-00019
- Dieker W, Derer J, Henzler T, et al. Pancreatitis, panniculitis and polyarthritis (PPP-) syndrome caused by post-pancreatitis pseudocyst with mesenteric fistula. Diagnosis and successful surgical treatment. Case report and review of literature. Int J Surg Case Rep. 2017;31:170-175. doi:10.1016/j.ijscr.2017.01.037
- Azar L, Chatterjee S, Schils J. Pancreatitis, polyarthritis and panniculitis syndrome. Joint Bone Spine. 2014;81(2):184. doi:10.1016/j.jbspin.2013.08.003
- Rodriguez M, Lopez GL, Prieto P, Fernandez L, Willisch A, Arce M. Massive subcutaneous and intraosseous fat necrosis associated with pancreatitis. Natural evolution of the radiographic picture. Clin Rheumatol. 1997;16(2):199-203. doi:10.1007/BF02247850
- Kotilainen P, Saario R, Mattila K, Nylamo E, Aho H. Intraosseous fat necrosis simulating septic arthritis and osteomyelitis in a patient with chronic pancreatitis. Arch Orthop Trauma Surg. 1998;118(3):174-175. doi:10.1007/s004020050342
- Harris MD, Bucobo JC, Buscaglia JM. Pancreatitis, panniculitis, polyarthritis syndrome successfully treated with EUS-guided cyst-gastrostomy. Gastrointest Endosc. 2010;72(2):456-458. doi:10.1016/j.gie.2009.11.040
- Gupta P, Saikia UN, Arora S, De D, Radotra BD. Panniculitis: a dermatopathologist's perspective and approach to diagnosis. Indian J Dermatopathol Diagn Dermatol. 2016;3(2):29. doi: 10.4103/2349-6029.195224
- Preiss JC, Faiss S, Loddenkemper C, Zeitz M, Duchmann R. Pancreatic panniculitis in an 88-year-old man with neuroendocrine carcinoma. Digestion. 2002;66(3):193-196. doi:10.1159/000066758
- Fine RM. The fine page. Subcutaneous fat necrosis, pancreatitis, and arthropathy. Int J Dermatol. 1983;22(10):575-576. doi:10.1111/j.1365-4362.1983.tb02128.x
- Shbeeb MI, Duffy J, Bjornsson J, Ashby AM, Matteson EL. Subcutaneous fat necrosis and polyarthritis associated with pancreatic disease. Arthritis Rheum. 1996;39(11):1922-1925. doi:10.1002/art.1780391121
- Dahl PR, Su WP, Cullimore KC, Dicken CH. Pancreatic panniculitis. J Am Acad Dermatol. 1995;33(3):413-417. doi:10.1016/0190-9622(95)91385-8
- Wilson HA, Askari AD, Neiderhiser DH, Johnson AM, Andrews BS, Hoskins LC. Pancreatitis with arthropathy and subcutaneous fat necrosis. Evidence for the pathogenicity of lipolytic enzymes. Arthritis Rheum. 1983;26(2):121-126. doi:10.1002/art.1780260201
- Loverdos I, Swan MC, Shekherdimian S, et al. A case of pancreatitis, panniculitis and polyarthritis syndrome: Elucidating the pathophysiologic mechanisms of a rare condition. J Pediatr Surg Case Rep. 2015;3(5):223-226. doi:10.1016/j.epsc.2015.03.014
- Yasuda MR, Roller LA, Fagenholz PJ, Hoang MP. Case 33-2020: A 55-Year-Old Man with Abdominal Pain, Joint Swelling, and Skin Lesions. N Engl J Med. 2020;383(17):1664-1671. doi:10.1056/NEJMcpc1916257