Abstract
Background
A 36-year-old woman with no risk factors for cancer was diagnosed with primary angiosarcoma of the breast.
Summary
After noting an enlarging right breast mass on self-exam, a 36-year-old woman presented to the clinic for further evaluation. She had no significant past medical history or risk factors for cancer. After a slight delay in workup, initial imaging was suspicious for a fibroadenoma, and core needle biopsy was suggestive of a vascular lesion. Excisional biopsy revealed a primary breast angiosarcoma. She subsequently underwent a right total mastectomy, demonstrating 1 cm of residual disease and widely negative margins. No additional therapy was recommended after surgery. Although the prognosis is poor generally and local recurrence rates are high, the patient was disease-free by exam and imaging at her one-year follow-up. While this case represents a classic presentation of primary angiosarcoma of the breast, it is rarely encountered clinically, making it a particularly challenging disease to diagnose and treat appropriately.
Conclusion
Compared to traditional breast cancer, primary angiosarcoma of the breast is rare, and the appropriate treatment strategy is not well studied. Here we present a case highlighting its presentation and the importance of surgery in its management.
Key Words
breast angiosarcoma; primary breast angiosarcoma; mastectomy
Case Description
Angiosarcoma is a malignant vascular neoplasm and the most common sarcoma of the breast.1,2 The most prevalent form arises in the dermis secondary to high-dose radiation exposure. However, a primary form can arise within the breast parenchyma.2 Primary angiosarcomas of the breast are rare tumors with a uniformly poor prognosis and ill-defined treatment guidelines. Here we report the case of a young female with an enlarging breast mass, subsequently diagnosed with primary breast angiosarcoma.
A healthy 36-year-old woman initially presented to her gynecologist with a self-palpated right breast mass. She described the mass as "squishy" and "grape-size." The patient denied any personal risk factors for breast cancer or significant family history. On physical exam, her provider could not definitively confirm the mass, and clinical follow-up was recommended.
Over the next six months, the patient reported enlargement of the mass and slight tenderness. A right breast ultrasound was suggestive of a 1.5 cm fibroadenoma. One month later, a diagnostic mammogram demonstrated diffusely increased parenchymal density and skin thickening but no dominant mass. Repeat ultrasound demonstrated a 5 cm hyperechoic area with a 1.2 cm central area of hypoechogenicity, a series of nodules, and "intense surrounding blood flow" (Figure 1). Core needle biopsy revealed a vasoformative lesion, favoring a hemangioma. In the operative note of the subsequent excisional biopsy, "extremely large vessels circumferentially around the mass" were noted. After a pathologic review locally and at a tertiary academic medical center, the final diagnosis was a well-differentiated angiosarcoma of the breast, which measured 1.2 cm by gross examination. Although the surgical margins were not specifically addressed, completion mastectomy was recommended.
Figure 1. Targeted Ultrasound of Right Breast Taken before Biopsy. Published with Permission
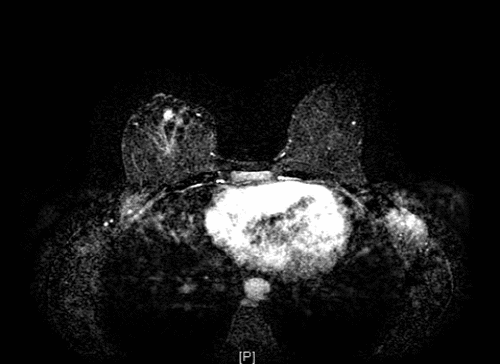
The patient was referred to a tertiary academic medical center for further management. A breast MRI was suggestive of residual disease (Figure 2), and a CT of the chest was negative for distant metastases. After a review of her case at the institution's multidisciplinary sarcoma tumor board, the consensus was to pursue definitive surgical resection with no adjuvant therapies. She underwent completion mastectomy, which demonstrated 1 cm of residual disease adjacent to the prior surgical site (Figure 3) and widely negative margins (closest > 1 cm). Interval surveillance imaging and breast/chest wall exams remained negative until two years postoperatively. While undergoing evaluation for breast reconstruction, she was noted to have an enlarging left pleural nodule and subsequently underwent wedge resection—pathology was consistent with metastatic angiosarcoma. Given this isolated recurrence on imaging, the multidisciplinary recommendation was to continue surveillance imaging, which has remained negative to date.
Figure 2. Breast MRI Demonstrating 1 cm of Enhancement Adjacent to Surgical Cavity in Right Breast, Favored Being Residual Angiosarcoma. Published with Permission
Figure 3. Representative Pathology Following Complete Resection. Published with Permission
Discussion
Angiosarcoma of the breast is a vascular tumor arising from mesenchymal tissue.1,2 Primary and secondary angiosarcomas of the breast share similar histopathology but are recognized for their distinct pathophysiology and presentation. However, the rarity of both diseases makes it difficult to analyze them separately, and most studies combine the diseases in their reports.
Compared to radiation-associated angiosarcoma, primary angiosarcoma of the breast has a younger onset and is typically diagnosed in women aged 30–40.2-4 As in the case above, it often presents as a painless, ill-defined mass and can be difficult to distinguish from fibroadenomas on exam.2,4 However, angiosarcomas demonstrate progressive growth, and most diagnoses begin with abnormal imaging. Retrospective studies have shown that many women with primary angiosarcoma of the breast have lung metastases at the time of presentation,2 and as such, chest imaging is a critical component of the diagnostic workup.
With its low incidence, the management of primary breast angiosarcoma is poorly defined and largely based on retrospective case studies. Among the treatment modalities evaluated, only wide surgical resection has been shown to significantly prolong survival in localized angiosarcoma.5 A total mastectomy is often recommended to obtain the necessary wide margins (> 3 cm).2,6 In patients who have undergone previous excisional biopsy with positive/close margins, such as our patient, the original biopsy site must also be removed. Contrary to traditional breast cancer (invasive ductal or lobular carcinoma), angiosarcoma of the breast demonstrates hematogenous spread, and lymph node metastasis is extremely rare.2,6 As such, there is no staging or survival benefit to lymph node sampling.
Despite surgery, angiosarcoma of the breast has a poor prognosis with an estimated five-year overall survival of 30% to 40%.6,7 Median overall survival has been reported to range from 1.1 to 2.8 years.2,3 Large tumor size (>5 cm) and positive margins are poor prognostic indicators.2 Even when negative margins are achieved, there is a high risk of locally and distant recurrence.2,5,8,9 In a retrospective case series of 41 women with primary breast angiosarcoma, a 24.4% local recurrence rate (median follow-up 36 months) was observed.10
Generally, there are no clear guidelines for reconstruction for breast angiosarcoma. Given that most breast angiosarcomas are secondary to radiation and have a significant cutaneous component,11 it is generally recommended to remove as much of the radiated tissue and skin as possible. This strategy contrasts most surgical techniques utilized in immediate breast reconstruction, which rely on preserving as much breast skin as possible (e.g., skin- and/or nipple-sparing mastectomy). In addition, reconstruction may compromise adjuvant treatments and subsequent exams. Surgical complications related to reconstruction may also arise, and as such, Crosby et al. concluded that delayed reconstruction might be preferred.12 However, a small retrospective analysis of 34 patients with breast angiosarcoma reported that many women received reconstruction (53.8% of those undergoing mastectomy) with no significant impact on survival.13 Consequently, the decision to pursue reconstruction should be made on a case-by-case basis, following a discussion between the surgeon and the patient. Although our patient elected to forego immediate reconstruction, she is considering delayed reconstruction.
The role of adjuvant therapies is controversial due to the lack of randomized clinical trials. Retrospective analyses suggest that some patients benefit from adjuvant radiation, namely those with positive margins or large tumors.1 One retrospective review of angiosarcomas, not limited to the breast, demonstrated that radiation therapy conferred no survival benefit in patients with localized disease.5 Accordingly, the multidisciplinary consensus at our institution did not favor adjuvant radiation for our patient. Given the high recurrence rates, close surveillance with imaging and chest wall exams is vital. Many providers follow the NCCN guidelines for Soft Tissue Sarcoma to guide surveillance.2,14
Conclusion
Primary angiosarcoma of the breast is a rare disease with a generally poor prognosis, in which surgery plays a critical role in treating localized disease. This classic case of primary breast angiosarcoma highlights the important tenets of its surgical management, including wide surgical margins.
Lessons Learned
Primary angiosarcoma of the breast is a rare, aggressive tumor with poorly defined treatment guidelines. Upfront surgical resection with wide margins is the only potentially curative treatment for localized disease. However, patients remain at high risk of local and distant recurrence necessitating close surveillance with imaging and clinical exams.
Authors
Rhodin KE; Plichta JK
Author Affiliations
Department of Surgery, Duke University Medical Center, Durham, NC 27710
Corresponding Author
Jennifer Plichta, MD, MS, FACS
Department of Surgery
Duke University Medical Center
DUMC 3513
Durham, NC 27710
Email: jennifer.plichta@duke.edu
Disclosure Statement
The authors have no conflicts of interest to disclose.
Funding/Support
The authors have no relevant financial relationships or in-kind support to disclose.
Acknowledgments
Dr. Allison Hall (Duke University Hospital) assisted in preparing the pathology slides figures.
Received: October 31, 2020
Revision received: December 27, 2020
Accepted: February 1, 2021
References
- Ghareeb ER, Bhargava R, Vargo JA, Florea AV, Beriwal S. Primary and Radiation-induced Breast Angiosarcoma: Clinicopathologic Predictors of Outcomes and the Impact of Adjuvant Radiation Therapy. Am J Clin Oncol. 2016;39(5):463-467. doi:10.1097/COC.0000000000000077
- Duncan MA, Lautner MA. Sarcomas of the Breast. Surg Clin North Am. 2018;98(4):869-876. doi:10.1016/j.suc.2018.03.013
- Vorburger SA, Xing Y, Hunt KK, et al. Angiosarcoma of the breast. Cancer. 2005;104(12):2682-2688. doi:10.1002/cncr.21531
- Scow JS, Reynolds CA, Degnim AC, Petersen IA, Jakub JW, Boughey JC. Primary and secondary angiosarcoma of the breast: the Mayo Clinic experience. J Surg Oncol. 2010;101(5):401-407. doi:10.1002/jso.21497
- Buehler D, Rice SR, Moody JS, et al. Angiosarcoma outcomes and prognostic factors: a 25-year single institution experience. Am J Clin Oncol. 2014;37(5):473-479. doi:10.1097/COC.0b013e31827e4e7b
- Hui A, Henderson M, Speakman D, Skandarajah A. Angiosarcoma of the breast: a difficult surgical challenge. Breast. 2012;21(4):584-589. doi:10.1016/j.breast.2012.01.001
- Donnell RM, Rosen PP, Lieberman PH, et al. Angiosarcoma and other vascular tumors of the breast. Am J Surg Pathol. 1981;5(7):629-642. doi:10.1097/00000478-198110000-00005
- Masai K, Kinoshita T, Jimbo K, Asaga S, Hojo T. Clinicopathological features of breast angiosarcoma. Breast Cancer. 2016;23(5):718-723. doi:10.1007/s12282-015-0630-y
- Gervais MK, Burtenshaw SM, Maxwell J, et al. Clinical outcomes in breast angiosarcoma patients: A rare tumor with unique challenges. J Surg Oncol. 2017;116(8):1056-1061. doi:10.1002/jso.24780
- Nascimento AF, Raut CP, Fletcher CD. Primary angiosarcoma of the breast: clinicopathologic analysis of 49 cases, suggesting that grade is not prognostic. Am J Surg Pathol. 2008;32(12):1896-1904. doi:10.1097/PAS.0b013e318176dbc7
- Kaklamanos IG, Birbas K, Syrigos KN, Vlachodimitropoulos D, Goutas N, Bonatsos G. Breast angiosarcoma that is not related to radiation exposure: a comprehensive review of the literature. Surg Today. 2011;41(2):163-168. doi:10.1007/s00595-010-4341-x
- Crosby MA, Chike-Obi CJ, Baumann DP, et al. Reconstructive outcomes in patients with sarcoma of the breast. Plast Reconstr Surg. 2010;126(6):1805-1814. doi:10.1097/PRS.0b013e3181f5276f
- Toesca A, Spitaleri G, De Pas T, et al. Sarcoma of the breast: outcome and reconstructive options. Clin Breast Cancer. 2012;12(6):438-444. doi:10.1016/j.clbc.2012.09.008
- National Comprehensive Cancer Network. Soft Tissue Sarcoma. NCCN Guidelines 2019; Version 2.2019. Accessed March 7, 2019. https://www.nccn.org/professionals/physician_gls/PDF/sarcoma.pdf