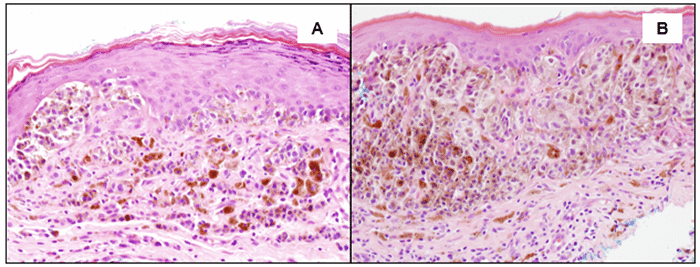
A) Confluent proliferation of atypical melanocytic cells along the base of the epidermis, a very minor superficial dermal component (towards the right), and numerous heavily pigmented melanophages (histiocytes) in the superficial dermis—magnification at 200x, H and E stain; B) Marked confluent proliferation of atypical melanocytes arranged in nests along the dermal/epidermal junction, many with intracytoplasmic melanin pigment—magnification at 200x, H and E stain
The patient was taken to the operating room for wide local excision of the melanoma biopsy site with 1 cm margins. This included an elliptical incision that was deepened down through the subcutaneous tissues to the level of the fascia. Once the specimen was removed, we raised small flaps to allow primary closure. The wound was closed with vertical mattress 3-0 Vicryl sutures. We elected to use Vicryl instead of Chromic sutures for patient comfort and to avoid suture removal in the clinic. No residual melanoma was identified on microscopic examination. The patient's recovery was uneventful, with excellent wound healing.
Discussion
Vulvovaginal malignant melanoma is an uncommon aggressive disease with a high rate of recurrence. In the United States, 1059 vulvar melanomas were diagnosed in a 30-year period (Surveillance, Epidemiology, and End Result Database 1973-2010).4,5 Postmenopausal women have the highest incidence with an average age of diagnosis at 61.6 years.3,5,6 The etiology for vulvovaginal melanoma is unknown. There have been reports of melanocytes found in the basal layer of the vaginal epithelium in healthy women, which is an embryological remnant of neural crest cells.4,7 Aberrantly located melanocytes from the vaginal epithelium are thought to be the cause of primary vaginal melanoma. Although there is no clear association with ultraviolet exposure, vulvar melanoma is more common in Caucasian women.1,5–7 Patients commonly present with a vulvar lump or mass and bleeding, pain, or itching.3,5–7 Melanomas occur slightly more frequently on the labia majora than labia minora. Patients can be asymptomatic, and lesions can be amelanotic, resulting in delayed presentation. Advanced age, increased Breslow thickness, and presence of lymphadenopathy affect overall survival.3,5
Diagnosis is confirmed by a full-thickness excisional biopsy of the entire lesion.1 If the entire lesion cannot be removed due to surrounding vital structures, an incisional biopsy can be an alternative. Immunohistochemical staining with HMB-45, S-100, Melan-A, and MART-1 can help to differentiate melanoma from other pigmented vulvar lesions.1,5,6 Clinical workup is similar to cutaneous melanoma with imaging indicated for stage IIIB or higher disease1, although Leitao et al.6 suggest pelvic imaging for all cases due to the disease's aggressive nature. Breslow thickness and AJCC staging are predictive of disease survival and recurrence; however, there is no consensus on a staging system for vulvar melanoma.5,7,8 The most recent AJCC staging guidelines include vulvar melanomas with cutaneous melanomas and not as vulvar cancers (squamous cell carcinoma).9
Surgical treatment of vulvar melanoma is WLE with negative margins.5–7 There is no survival benefit between WLE and radical vulvectomy. There is no clear consensus on recurrence rates.6,7,10 Data on optimal surgical margin for vulvar melanoma is also lacking, with current guidelines similar to cutaneous melanoma. Irvin et al. 7 determined that a margin wider than 2 cm did not improve survival. Evidence on the benefit of sentinel lymph node biopsy in vulvar melanoma is likewise scarce. The current indication for sentinel lymph node biopsy coincides with cutaneous melanoma, although the clinical benefit of completion lymphadenectomy after a positive sentinel lymph node biopsy is unknown.6
Neoadjuvant chemotherapy and radiation have been studied to decrease tumor bulk prior to surgical resection. Adjuvant radiation is used to control local disease for positive margins and lymph nodes.6,7 Systemic therapy for an unresectable disease should include molecular testing to direct immunotherapy and targeted therapy, specifically analyses of c-KIT, BRAF, and NRAS mutations.1,5,6 The reported 5-year survival rate of vulvar melanoma ranges from 10 to 63 percent.5,8 In a cohort study of 51 patients with vulvar melanoma, 32 patients recurred with 58 percent locoregional, 28 percent at distant sites, and 19 percent at both local and distant sites.11 Average time to local recurrence is 5.25 years.1 Older patients have an increased risk of recurrence.
Our unique case emphasizes the importance of early diagnosis of vulvar melanoma, given that the patient's melanoma was identified at an early stage and occurred at a much younger age than is typical. Delayed diagnosis contributes to poor survival and poor quality of life. Surgery is the primary treatment, and our patient was able to undergo WLE with 1 cm margins without radical resection of major anatomical structures. Close surveillance is indicated due to the high recurrence rate of vulvar melanoma, even with early-stage disease. Since there is no research or consensus on an appropriate follow-up schedule, the regimen for vulvar invasive squamous cell carcinoma has been adopted for vulvar melanoma. This surveillance schedule consists of physical examinations every 3–4 months for the first two years after diagnosis and biannually in the third through fifth years. Long-term follow-up recommendations are desperately needed in the field as recurrences after five years are unfortunately prevalent.
Conclusion
Vulvar malignant melanoma is an uncommon and aggressive disease with high rates of recurrence. Surgery is the primary treatment: WLE with 1 cm margins. Surveillance consists of physical examinations every 3–4 months for the first two years after diagnosis and biannually in the third through fifth years. However, long-term recommendations are needed in the field as recurrence after five years is frequent.
Lessons Learned
Early diagnosis in vulvar melanoma improves survival. Breslow thickness and AJCC staging, adapted from cutaneous melanoma, are predictive of survival and recurrence, but there is no consensus on a staging system. Surgery is the primary treatment, and indications for sentinel lymph node biopsy follow those of cutaneous melanoma. Recommendations for long-term surveillance are urgently needed.
Authors
Chen Ca; Schwartz MRb; Holder AMa
Author Affiliations
- Department of Surgery, Houston Methodist Hospital, Houston, TX 77030
- Department of Pathology and Genomic Medicine, Houston Methodist Hospital, Houston, TX 77030
Corresponding Author
Ashley M. Holder, MD, FACS
Division of Surgical Oncology
University of Alabam at Birmingham
1808 7th Avenue South, BDB 571
Birmingham, AL 35294
E-mail: amholder@uab.edu
Disclosure Statement
The authors have no conflicts of interest to disclose.
Funding/Support
The authors have no financial relationships or in-kind support to disclose.
Received: July 18, 2020
Accepted for Publication: September 17, 2020
References
- Kalampokas E, Kalampokas T, Damaskos C. Primary vaginal melanoma, a rare and aggressive entity. A case report and review of the literature. In Vivo. 2017;31(1):133-139. doi:10.21873/invivo.11036
- Lerner BA, Stewart LA, Horowitz DP, Carvajal RD. Mucosal melanoma: new insights and therapeutic options for a unique and aggressive disease. Oncology (Williston Park). 2017;31(11):e23-e32. Published 2017 Nov 15.
- Mihajlovic M, Vlajkovic S, Jovanovic P, Stefanovic V. Primary mucosal melanomas: a comprehensive review. Int J Clin Exp Pathol. 2012;5(8):739-753.
- Sanchez A, Rodríguez D, Allard CB, et al. Primary genitourinary melanoma: Epidemiology and disease-specific survival in a large population-based cohort. Urol Oncol. 2016;34(4):. doi:10.1016/j.urolonc.2015.11.009
- Boer FL, Ten Eikelder MLG, Kapiteijn EH, Creutzberg CL, Galaal K, van Poelgeest MIE. Vulvar malignant melanoma: Pathogenesis, clinical behaviour and management: Review of the literature. Cancer Treat Rev. 2019;73:91-103. doi:10.1016/j.ctrv.2018.12.005
- Leitao MM Jr, Cheng X, Hamilton AL, et al. Gynecologic Cancer InterGroup (GCIG) consensus review for vulvovaginal melanomas. Int J Gynecol Cancer. 2014;24(9 Suppl 3):S117-S122. doi:10.1097/IGC.0000000000000198
- Irvin WP Jr, Bliss SA, Rice LW, Taylor PT Jr, Andersen WA. Malignant melanoma of the vagina and locoregional control: radical surgery revisited. Gynecol Oncol. 1998;71(3):476-480. doi:10.1006/gyno.1998.5188
- Trimble EL, Lewis JL Jr, Williams LL, et al. Management of vulvar melanoma. Gynecol Oncol. 1992;45(3):254-258. doi:10.1016/0090-8258(92)90300-8
- Gershenwald JE, Scolyer RA. Melanoma staging: American Joint Committee on Cancer (AJCC) 8th edition and beyond [published correction appears in Ann Surg Oncol. 2018 Dec;25(Suppl 3):993-994]. Ann Surg Oncol. 2018;25(8):2105-2110. doi:10.1245/s10434-018-6513-7
- Ragnarsson-Olding BK. Primary malignant melanoma of the vulva--an aggressive tumor for modeling the genesis of non-UV light-associated melanomas. Acta Oncol. 2004;43(5):421-435. doi:10.1080/02841860410031372
- Verschraegen CF, Benjapibal M, Supakarapongkul W, et al. Vulvar melanoma at the M. D. Anderson Cancer Center: 25 years later. Int J Gynecol Cancer. 2001;11(5):359-364. doi:10.1046/j.1525-1438.2001.01043.x