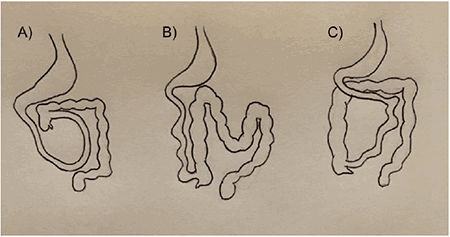
Figure 5: Types of malrotation: (A) depicts incomplete rotation, (B) depicts reverse rotation, (C) depicts nonrotation.
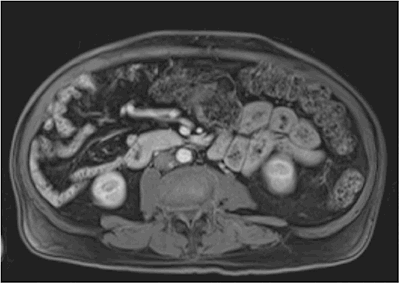
In incomplete rotation, the duodenum is incompletely rotated and the ligament of Treitz is either absent or to the right of the midline. The colon can also be incompletely rotated with the formation of Ladd’s bands. In non-rotation, the patient has a rotation of less than 90° with no ligament of Treitz as well as a non-fixed cecum. In reverse rotation, which is the most rare, the gut is rotated in a clockwise fashion. According to these categories, our patient had a non-rotation malrotation of the intestine with the duodenum right to the midline and high lying cecum which predisposes to volvulus, especially in the presence of Ladd’s bands (Figure 3). He had some fibrous adhesions from the cecum toward the right abdominal wall, kinking the duodenum where it would normally pass under the ligament of Treitz, but instead headed toward his right side (Figure 3). This was not completely obstructing and did not tether the cecum, likely explaining why he never presented acutely with an obstruction during his 84 years of life.
When adults are diagnosed with malrotation they have a variety of presentations ranging from acute bowel obstruction as seen in children, to a more indolent course.4-6 Patients can be completely asymptomatic or just have a mild and chronic abdominal pain.1 Interestingly, some patients’ only symptomology is gastrointestinal reflux disease, which was seen in our patient.1 It has been reported that some adults can have a long history of mild abdominal pain followed by an acute volvulus obstructive episode.16 Unfortunately, many of these symptoms are neither sensitive nor specific for intestinal malrotation, resulting in a difficulty to diagnose adults with malrotation. Because of this, it is difficult to say for certain whether or not our patient’s past medical history of GERD was related to his malrotation, but it is entirely possible. The patient’s presenting symptoms, however, can more easily be attributed to his pancreatic tumor. The diagnosis of malrotation in an adult with chronic abdominal pain can be established with an upper GI series, CT scan, or other abdominal imaging documenting a duodenum that does not traverse the midline or a pathognomonic whirl pattern around the SMA.17
In adults, the treatment of malrotation depends on the acuity of illness and ranges from emergent laparotomy with hemodynamic resuscitation to a total lack of treatment across a lifetime due to the condition being asymptomatic. Chronic symptoms can be treated surgically with an elective Ladd procedure, in which the surgeon assesses for volvulus with counterclockwise detorsion, and divides the Ladd band and inter-mesenteric band between loops of bowel. The Ladd procedure then proceeds with a prophylactic appendectomy due to the aberrant location of the appendix to prevent future confusion. Our patient was asymptomatic from his malrotation. The subtle Ladd’s bands were taken down during the mobilization and resection of the duodenum. Given his lack of symptoms and age, appendectomy and fixation of the cecum were not performed. A six-year review determined that close observation is acceptable in asymptomatic or mildly symptomatic patients, as in our patient with reflux disease.18 A decision analysis actually reports that there is an increased advantage of observation after the second decade of life and that the rare occurrence of midgut volvulus does not justify a prophylactic Ladd procedure in most adults.19
While the reason surgery was performed was for his glucagon-producing PNET, the emphasis of this review is on malrotation and its management during a Whipple operation. However, we found it interesting to have diagnosed and managed both of these rare entities in one patient. There have been several reported cases of pancreaticoduodenectomy in the setting of malrotation, though none for PNET. In all reported cases, the patients lacked a ligament of Treitz. Saito and colleagues report a 74 year old male patient with cholangiocarcinoma treated with pancreaticoduodenectomy.10 Unlike our patient, this patient had an SMV rotation sign, in which the SMV was left of the SMA. His postoperative course was uneventful. Another case of malrotation in the setting of pancreaticoduodenectomy was described in a 59 year-old male patient with common bile duct cancer.11 This particular patient had levocardia, malrotation, and situs ambiguous, in which the patient had a right-sided stomach and spleen, midline liver, and multiple vascular variations around the celiac axis. His postoperative course was also uncomplicated and surgery was successful. Kawahara reports a 63 year-old patient with incomplete fixation malrotation and a circulatory disorder of the small intestine making dissection very difficult.12
A review of these cases and report of a patient with pancreatic adenocarcinoma cautions the necessity of understanding the underlying vascular variants in association with malrotation.13 A vascular variant has been described in a 61 year-old male with common bile duct cancer in which the patient had a replaced common hepatic artery arising from a branch from the SMA, which coursed through the pancreas.14 The surgical team for this case removed the branches to the pancreas, but emphasized the importance of preserving blood flow to the liver. Lastly, three additional cases of pancreaticoduodenectomy within the setting of malrotation are recorded in which each patient had variations in arterial and venous supply around the celiac and mesenteric vessels, respectively.20 Outcomes in these patients were again favorable due to diligent dissection and identification of vascular structures before parenchymal division and vessel ligation. The common theme among these reports holds that diligent vascular dissection is necessary during pancreaticoduoenectomy in the setting of intestinal malrotation. Our patient had normal vascular anatomy.
Conclusion
To conclude, this is a rare case of asymptomatic malrotation in an elderly patient diagnosed during a Whipple operation for a glucagon-producing PNET. As his malrotation was an incidental finding, the subtle Ladd’s bands were taken down in the course of the operation, and he had made it to the age of 84 with minimal symptomology, the decision to perform a concomitant formal Ladd procedure was deferred. In this case, the lack of a ligament of Treitz actually facilitated resection and reconstruction and no vascular anomalies were noted. However, variations in bowel and vascular anatomy seen in patients with intestinal malrotation require caution and care on the part of the surgical team during dissection.
Lessons Learned
Asymptomatic malrotation can be missed on imaging. Pancreaticoduodenectomy in malrotated patients does not require takedown of the ligament of Treitz or bringing the jejunum retrocolic for reconstruction. While surgical dissection should be prepared for possible vascular anomalies, a concomitant prophylactic Ladd procedure is unlikely justified in the asymptomatic elderly.
Authors
Nina S. Pollack, BA
Department of Surgery
University of Arizona College of Medicine, Tucson, AZ
Kelvin Memeh, MD
Department of Surgery
University of Arizona College of Medicine, Tucson, AZ
Taylor S. Riall, MD, PhD
Department of Surgery
University of Arizona College of Medicine, Tucson, AZ
Correspondence Author
Taylor S. Riall, MD, PhD
1501 N Campbell Ave
Tucson, AZ 85724
520-626-2635
tsriall@surgery.arizona.edu
Author Contributions
All authors participated in the case.
NSP drafted the report and did an extensive literature review.
TSR and KM critically revised the draft and reviewed the literature.
Disclosure Statement
The authors whose names are listed immediately above certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
References
- von Flüe M, Herzog U, Ackermann C, Tondelli P, Harder F. Acute and chronic presentation of intestinal nonrotation in adults. Dis Colon Rectum. 1994 Feb;37(2):192-8.
- Gamblin TC, Stephens RE Jr, Johnson RK, Rothwell M. Adult malrotation: a case report and review of the literature. Curr Surg. 2003 Sep-Oct;60(5):517-20.
- Yanez R, Spitz L. Intestinal malrotation presenting outside the neonatal period. Arch Dis Child.1986 Jul;61(7):682-5.
- Devlin HB, Williams RS, Pierce JW. Presentation of midgut malrotation in adults. Br Med J. 1968 Mar 30;1(5595):803-7.
- Buchmiller T. Intestinal malrotation in adults. In: UpToDate, Post TW (Ed), UpToDate, Waltham, MA. (Accessed on July 24, 2017.)
- Durkin ET, Lund DP, Shaaban AF, Schurr MJ, Weber SM. Age-related differences in diagnosis and morbidity of intestinal malrotation. J Am Coll Surg. 2008 Apr;206(4):658-63.
- Schluman J, Edmonds LE, McClearn AB, Jensvold N, Shaw GM. Surveillance for and comparison of birth defect prevalences in two geographic areas - Unites States, 1983–1988. MMWR Morb Mort Wkly Rep. 1993;42:1–8.
- Dietz DW, Walsh RM, Grundfest-Broniatowski S, Lavery IC, Fazio VW, Vogt DP. Intestinal malrotation: a rare but important cause of bowel obstruction in adults. Dis Colon Rectum 2002;45:1381-6.
- Jensen RT, Cadiot G, Brandi ML, et al. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms: functional pancreatic endocrine tumor syndromes. Neuroendocrinology. 2012;95(2):98-119. Epub 2012 Feb 15
- Saito Y, Miyamoto A, Maeda S, et al. A case of cholangiocarcinoma with intestinal malrotation treated with pancreaticoduodenectomy. Gan To Kagaku Ryoho. 2015 Nov;42(12): 1729-31. Japanese
- Lim HK, Choi YS, Lee SE, Kang H. Pancreaticoduodenectomy performed in a patient with situs ambiguous accompanied with isolated levocardia, malrotation, and normal spleen. Ann Surg Treat Res. 2014 Dec;87(6):340-4.
- Kawahara R, Horiuchi H, Nogita H, et al. A case of cancer of the ampulla of Vater accompanied by malrotation. Kurume Med J. 2013;60(1):33-6.
- Plackett TP, Takamori R, Izawa M. Pancreaticoduodenectomy in the setting of intestinal malrotation. Hawaii Med J. 2011 Nov;70(11):237-8.
- Hayashi T, Takano S, Kimura F, et al. A Case of cholangiocarcinoma with hepatomesenteric trunk and intestinal malrotation treated with pancreaticoduodenectomy. Gan To Kagaku Ryoho. 2010 Nov;37(12):2723-5. Japanese.
- Panksy, Ben. Review of Medical Embryology. New York: Macmillan, 1982. Print.
- Fung AT, Konkin DE, Kanji ZS. Malrotation with midgut volvulus in an adult: a case report and review of the literature. J Surg Case Rep. 2017 May 10;2017(5):rjx081.
- Shahverdi E, Morshedi M, Allahverdi Khani M, Baradaran Jamili M, Shafizadeh Barmi F. Utility of the CT scan in diagnosing midgut volvulus in patients with chronic abdominal pain. Case Rep Surg. 2017;2017:1079192.
- McVay MR, Kokoska ER, Jackson RJ, Smith SD. Jack Barney Award. The changing spectrum of intestinal malrotation: diagnosis and management. Am J Surg. 2007 Dec;194(6):712-7.
- Malek MM, Burd RS. The optimal management of malrotation diagnosed after infancy: a decision analysis. Am J Surg. 2006 Jan;191(1):45-51.
- Mateo R, Stapfer M, Singh G, Sher L, Jabbour N, Selby RR, Genyk Y. Pancreaticoduodenectomy in adults with congenital intestinal rotation disorders. Pancreas. 2005 Nov;31(4):413-5.