Discussion
ECMO represents an advanced modality of support for critically ill patients suffering from cardiac and/or pulmonary disease whereby medical therapies are limited or futile. The two forms of ECMO, VA-ECMO and VV-ECMO, provide an extracorporeal circuit to assist with oxygenation, gas exchange, and/or hemodynamic support. ECMO use is highly resource-intensive and requires advanced training of perfusionists, respiratory therapists, and nurses for proper use. Emerging clinical data demonstrates that the use of and indications for ECMO in clinical practice is growing. In fact, there has been an exponential growth in the number of ECMO programs over the last decade, with more than 400 ECMO centers worldwide.3 While large academic centers with organ transplantation services frequently employ ECMO as a bridge for recovery or transplantation of critically ill patients, the use of ECMO in smaller community hospitals remains largely unknown.
As the COVID-19 pandemic has spread, so, too, has the necessity for broadly distributing information about the virus. Clinical knowledge has been propagated widely via e-mail, popular media, and national societies. Well-established publication companies have nearly halved their manuscript processing times to expedite the publishing of SARS-CoV-2-related data. In this light, national and international organizations like ELSO have openly recruited for previously restricted society memberships to capture more virus-related data. This quest has been advantageous for small hospitals like ours with earlier established, underreported programs. While we have performed ECMO at this hospital for over 20 years, our first ELSO application is now pending.
The spread of SARS-CoV-2 across the world has been paralleled by significant resource limitations. Equipment like gloves, masks, ventilators, and even trained hospital staff have largely been insufficient. We were fortunate to have recognized the need to upgrade and standardize our ECMO program last year before the onslaught of the SARS-CoV-2 outbreak. At that time, we also established a collaboration with a large volume ELSO ECMO Center of Excellence in our region. As shown in this case report, the delivery of much-needed equipment occurred just in time. ECMO management has classically been the responsibility of perfusionists, but larger, experienced centers have been increasingly relying on nurses and respiratory therapists to assist in circuit maintenance. Due to resource limitations, we recognized the need to integrate nurses into our protocols as well. Working in concert with nurse educators, our surgeons have been able to develop simplified workflows and order sets necessary to facilitate this transition.
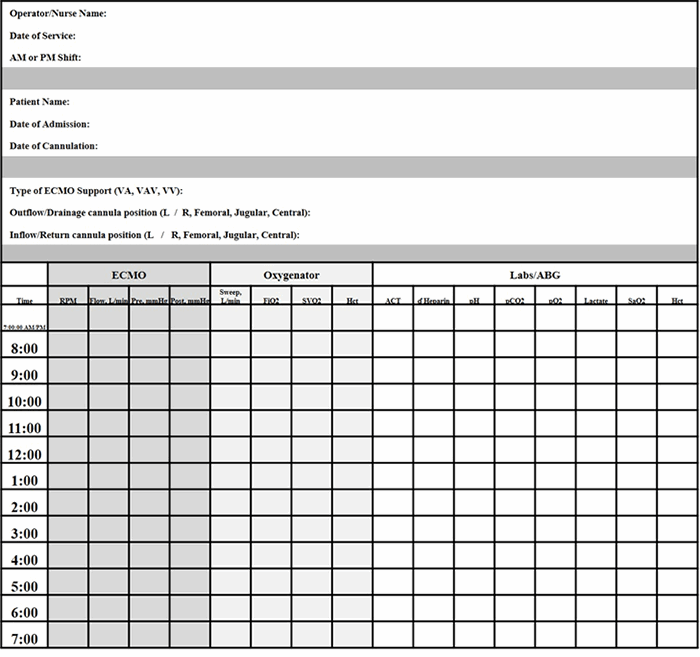
Many ECMO devices are commercially available. The management of VV-ECMO(compared to VA-ECMO) is also more simplified. With VV-ECMO, the speed of the pump is set to achieve flows needed to support the clinical status of the patient. Hemodynamic volatility can be less than VA-ECMO. Weaning is also performed by decreasing supplemental oxygenation (FiO2 of the circuit) or sweep, rarely interfering with blood flow, thus minimizing potential risks of thrombosis and embolism. Our simplified patient workflow allowed nurses to keep timely records of ventilator, ECMO, and laboratory parameters that could easily identify changes in clinical status. We supported our ECMO team nurses with 24-hour perfusionist and surgeon backup. Morning and afternoon rounds were completed with the surgeon, bedside nurse, and perfusionist to identify parameter goals and address any circuit or clinical concerns.
The application of ECMO in the care for critically ill patients suffering from COVID-19 is growing. Large academic hospitals, supported by transplant centers, routinely employ this technology under the direction of numerous multidisciplinary specialists. However, smaller community hospitals may have access to this technology—but possibly more antiquated processes for application. During this pandemic, the transfer of critically ill patients from smaller hospitals to larger medical centers was limited due to fears of viral spread. Before our hospital incorporated this technology, our ability to utilize ECMO in the care of even one ARDS patient would have compromised our ability to provide perfusion services to the rest of the hospital, thus suspending our entire cardiology service line. While large academic centers are reporting greater rates of survival of patients treated with VV-ECMO for ARDS due to COVID-19, this case highlights that current ECMO technologies can be safely and effectively adopted by care teams no matter the hospital size.
Conclusion
In summary, we presented a successful case of VV-ECMO utilized to treat a patient suffering from COVID-19. We have shown our patient selection criteria and patient care workflows that assisted with the rapid integration of nurses into our ECMO program.
Lessons Learned
This case shows how a small community hospital can quickly adopt innovative techniques for meeting the challenge of treating a unique subset of critically ill patients suffering from COVID-19.
Authors
Melvan JNa; Justicz Aa; Wang Ib; Dennis Ra; Farah Sa; Megano Na; Echarte Da; David Ia
Corresponding Author
John Nicholas Melvan, MD, PhD
Holy Cross Hospital
Division of Cardiothoracic Surgery
4725 N. Federal Highway, Suite 402
Fort Lauderdale, FL 33308
Phone: (954) 267-6770
E-mail: johnnicholas.melvan@holy-cross.com
Author Affiliations
a Division of Cardiothoracic Surgery, Holy Cross Hospital, Fort Lauderdale, FL
b Memorial Healthcare System, Fort Lauderdale, FL
Disclosure Statement
The authors have no conflicts of interest to disclose.
Acknowledgments
The authors would like to thank Holy Cross Hospital cardiovascular ICU nurses, who volunteered to participate in this ECMO team. We utilized their constructive feedback to build these protocols. The authors also thank Asser Guirgues of Abbott Laboratories for his industry-sponsored training.
References
- MacLaren G, Fisher D, Brodie D. Preparing for the Most Critically Ill Patients With COVID-19: The Potential Role of Extracorporeal Membrane Oxygenation. JAMA. 2020;323(13):1245-1246. doi:10.1001/jama.2020.2342
- Combes A, Hajage D, Capellier G, et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N Engl J Med. 2018;378(21):1965-1975. doi:10.1056/NEJMoa1800385
- Ramanathan K, Antognini D, Combes A, et al. Planning and provision of ECMO services for severe ARDS during the COVID-19 pandemic and other outbreaks of emerging infectious diseases. Lancet Respir Med. 2020;8(5):518-526. doi:10.1016/S2213-2600(20)30121-1