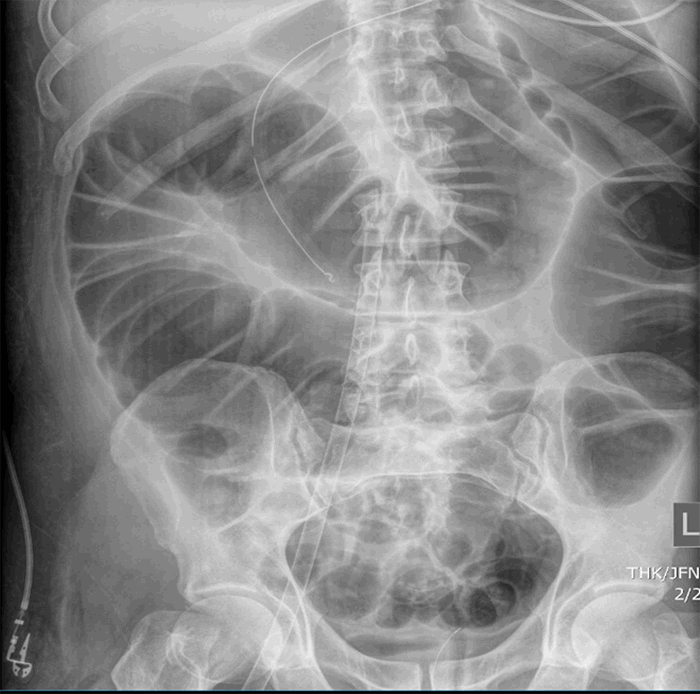
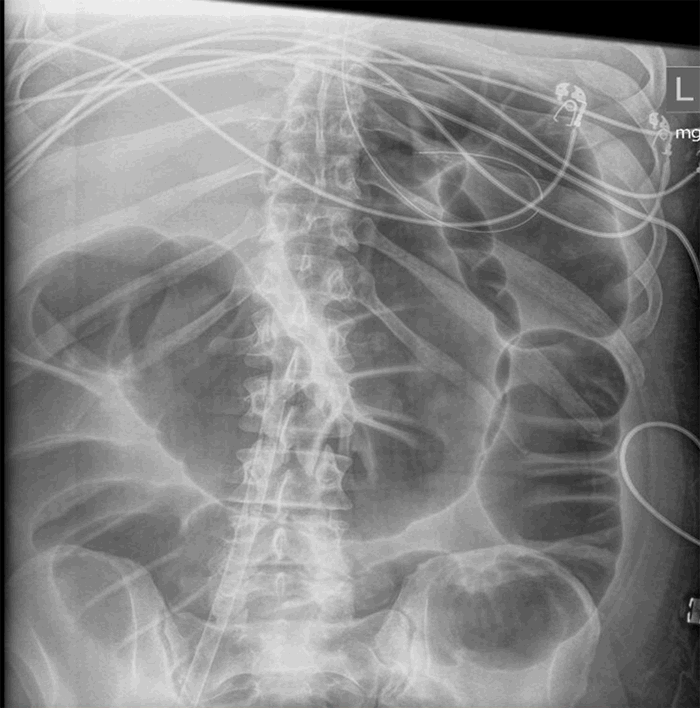
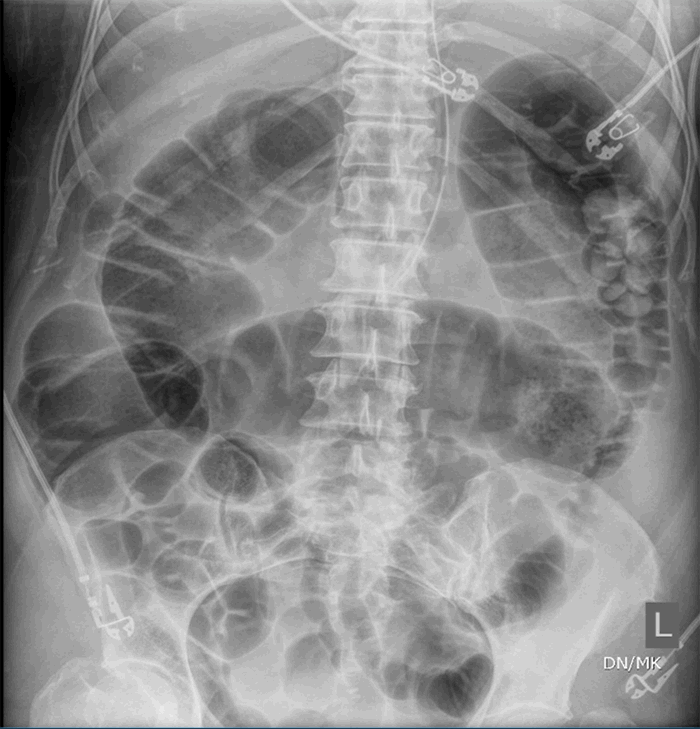
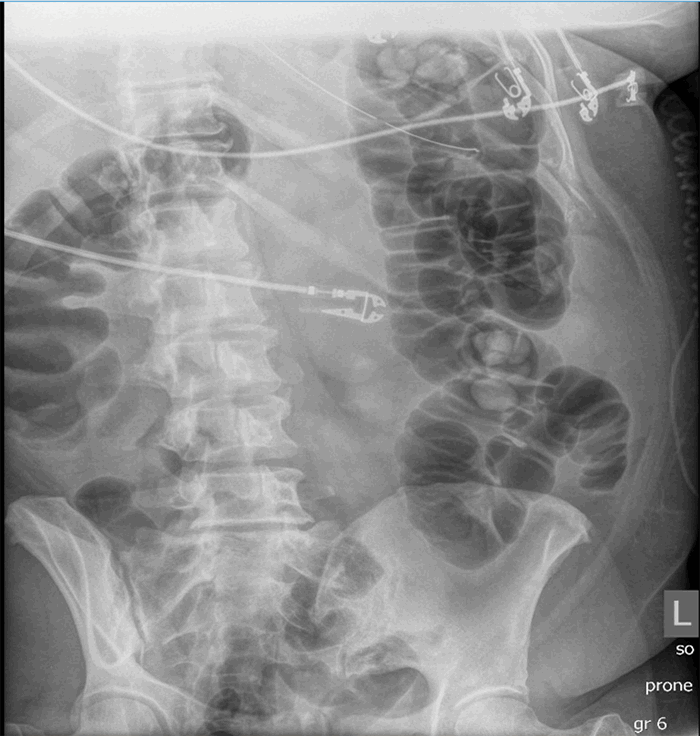
At the time of this paper’s creation, the patient continued to improve, approached being extubated, and tolerated tube feeds. She received subcutaneous Lovenox for anticoagulation.
Discussion
The presentation in these three patients is consistent with a paralytic ileus of the colon from COVID-19 resembling acute colonic pseudo-obstruction or Ogilvie syndrome. Ogilvie syndrome is a functional disorder that develops in patients with serious underlying diseases and is characterized by profound dilatation of the colon without a true mechanical obstruction.4,5 Its incidence in inpatients is estimated at 100 cases per 100,000 admissions with an overall mortality of eight percent.6–8 The incidence of colonic ischemia or perforation in these patients is about 15 percent, and when these occur, mortality rises to about 40 percent.6,9–11 Patients with the highest risks of perforation are those with a cecal diameter greater than 12 cm and colonic distension over six days.6 Predictors of mortality include increasing age, increasing cecal diameter, delay in decompressing the bowel, and whether ischemia or perforation is present.6 Options for management include initial nonoperative management (nothing by mouth, nasogastric tube placement, rectal tube placement), pharmacological decompression of the bowel with neostigmine (for patients who do not respond to conservative measures and without evidence of colonic ischemia or perforation), an aggressive bowel regimen, and endoscopic decompression with colonoscopy.12
It is unclear whether this paralytic ileus of the colon in our patients is a form of Ogilvie syndrome or if it is a unique manifestation of COVID-19 in the gastrointestinal tract. The association between neurotropic viral infections and chronic intestinal pseudo-obstruction (CIPO) has been previously described.13 Herpes viruses such as Epstein-Barr virus, varicella virus, John Cunningham (JC) virus, and cytomegalovirus have all been linked to intestinal pseudo-obstruction in the past. It is postulated that these viruses directly invade the myenteric plexus causing neurogenic impairment and subsequent intestinal impairment. Specifically, the JC viral DNA has been isolated in the myenteric plexus of 80 percent of affected patients with chronic idiopathic pseudo-obstruction compared with 9.7 percent of control patients.14 While CIPO predominantly affects the small intestine,15 the colonic ileus or pseudo-obstruction observed in our three patients was almost entirely colonic.
Coronaviruses are known to have neurotropic potential16,17 and molecular studies have shown that human coronaviruses can infect the central nervous system.18 In a recent case series of 214 patients with a confirmed diagnosis of COVID-19 infection, neurologic clinical features were present in 36.4 percent of patients.19 Thus, the possibility exists that the SARS-COV-2 virus could directly invade the myenteric plexus, resulting in severe neuroplegia that ultimately manifests as colonic paralytic ileus. This possibility deserves investigation.
The three patients presented in this case series have several similarities as well as distinct differences (Table 1). All were COVID-19 positive with severe ARDS requiring intubation and mechanical ventilation; one required ECMO. All patients had evidence of multiple organ dysfunction syndrome, and they also all had evidence of acute colonic pseudo-obstruction, with functional dilatation of the colon but no mechanical obstruction. The patient (described in Case 1) who developed cecal ischemia had a 2 cm increase in his cecal diameter over a 24-hour period, his cecal diameter with the first KUB was 11.6 cm, his WBC doubled within 24 hours, and his lactate level continued to rise. Pneumatosis of the colon was evident on the KUB before operative intervention. Although he responded initially to nonoperative measures like the placement of a rectal tube and an aggressive bowel regimen, he went on to develop bowel ischemia. The second patient (described in Case 2) who responded to nonoperative management had a colonic diameter of 7.8 cm on the initial KUB, his colonic diameter increased by approximately 0.5 cm per day, his WBC was on the low end of normal, and his lactate was normal. The third patient (described in Case 3) responded to nonoperative management and had normal lactate as well as WBC.
|
|
Case 1
|
Case 2
|
Case 3
|
|
Age
|
69
|
49
|
43
|
|
Gender
|
Male
|
Male
|
Female
|
|
BMI (kg/m2)
|
25.8
|
26.1
|
33.3
|
|
WBC (uL)
|
6900
|
4000
|
8400
|
|
Lymphocyte%
|
19.3
|
11.0
|
7.8
|
|
D-dimer (ng/mL)
|
458
|
995
|
1361
|
|
Lactate (mg/dL), at time of diagnosis of ileus
|
2.9
|
0.9
|
0.8
|
|
Fibrinogen (mg/dL)
|
1034
|
811
|
618
|
|
Abdominal X ray
|
Colonic dilatation
|
Colonic dilatation
|
Colonic dilatation
|
|
Rectal tube
|
Yes
|
Yes
|
Yes
|
|
Bowel ischemia
|
Yes
|
No
|
No
|
|
Surgical intervention
|
Yes
|
No
|
No
|
|
ARDS
|
Yes
|
Yes
|
Yes
|
|
Mortality
|
Yes
|
No
|
No
|
Table 1. Summary of the major findings in these three described case reports
It is difficult to generalize based on a case series of only three patients, but clinicians taking care of COVID-positive patients should be aware of the risk of colonic paralytic ileus in this disease and its potentially devastating consequences. It remains unclear whether neostigmine would be effective in these cases as we did not use it in any of the three patients in our series, but extrapolating from Ogilvie, we would recommend its use in case of worsening of the COVID-19 paralytic ileus despite adequate bowel regimen and colonic decompression with a rectal tube. As multi-institutional data accumulates on these patients from across the United States and the world, we should investigate further the epidemiology, etiology, predictors, and outcomes of COVID-19-related paralytic colonic ileus and pseudo-obstruction.
Conclusion
In the three cases reported, paralytic ileus was present, and it progressed to bowel ischemia. Front-line physicians caring for patients with COVID-19 infection should be aware of this complication.
Lessons Learned
Paralytic ileus involving both the small and the large bowel appears to be a complication of COVID-19 infection. The potential for this to progress to frank bowel ischemia exists. Aggressive bowel regimen and close abdominal exam are both warranted in this condition.
Authors
Hwabejire JO; Saillant N; King R; Velmahos GC; Kaafarani HMA
Corresponding Author
John O. Hwabejire, MD, MPH
Division of Trauma, Emergency Surgery, and Surgical Critical Care
Department of Surgery, Massachusetts General Hospital and Harvard Medical School, Boston, MA
Phone: (617) 643-2439
E-mail: jhwabejire@partners.org
Author Affiliations
Division of Trauma, Emergency Surgery, and Surgical Critical Care, Department of Surgery, Massachusetts General Hospital
Harvard Medical School, Boston, MA
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- Coronavirus Disease 2019 (COVID-19), Situation Report – 91. World Health Organization https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200812-covid-19-sitrep-205.pdf?sfvrsn=627c9aa8_2 Last accessed: 08/13/2020
- Coronavirus Disease 2019 (COVID-19), US Cases. The Centers for Disease Control and Prevention https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html Last accessed: 08/13/2020
- El Moheb M, Naar L, Christensen MA, et al. Gastrointestinal Complications in Critically Ill Patients With and Without COVID-19 [published online ahead of print, 2020 Sep 24]. JAMA. 2020;324(18):1899-1901. doi:10.1001/jama.2020.19400
- Hutchinson R, Griffiths C. Acute colonic pseudo-obstruction: a pharmacological approach. Ann R Coll Surg Engl. 1992;74(5):364-367.
- Saunders MD, Kimmey MB. Colonic pseudo-obstruction: the dilated colon in the ICU. Semin Gastrointest Dis. 2003;14(1):20-27.
- Wells CI, O'Grady G, Bissett IP. Acute colonic pseudo-obstruction: A systematic review of aetiology and mechanisms. World J Gastroenterol. 2017;23(30):5634-5644. doi:10.3748/wjg.v23.i30.5634
- Vanek VW, Al-Salti M. Acute pseudo-obstruction of the colon (Ogilvie's syndrome). An analysis of 400 cases. Dis Colon Rectum. 1986;29(3):203-210. doi:10.1007/BF02555027
- Ross SW, Oommen B, Wormer BA, et al. Acute Colonic Pseudo-obstruction: Defining the Epidemiology, Treatment, and Adverse Outcomes of Ogilvie's Syndrome. Am Surg. 2016;82(2):102-111. doi:10.1177/000313481608200211
- Nanni G, Garbini A, Luchetti P, Nanni G, Ronconi P, Castagneto M. Ogilvie's syndrome (acute colonic pseudo-obstruction): review of the literature (October 1948 to March 1980) and report of four additional cases. Dis Colon Rectum. 1982;25(2):157-166. doi:10.1007/BF02553265
- Delgado-Aros S, Camilleri M. Pseudo-obstruction in the critically ill. Best Pract Res Clin Gastroenterol. 2003;17(3):427-444. doi:10.1016/s1521-6918(03)00023-4
- Saunders MD, Kimmey MB. Colonic pseudo-obstruction: the dilated colon in the ICU. Semin Gastrointest Dis. 2003;14(1):20-27.
- Maloney N, Vargas HD. Acute intestinal pseudo-obstruction (Ogilvie's syndrome). Clin Colon Rectal Surg. 2005;18(2):96-101. doi:10.1055/s-2005-870890
- De Giorgio R, Ricciardiello L, Naponelli V, et al. Chronic intestinal pseudo-obstruction related to viral infections. Transplant Proc. 2010;42(1):9-14. doi:10.1016/j.transproceed.2009.12.014
- Selgrad M, De Giorgio R, Fini L, et al. JC virus infects the enteric glia of patients with chronic idiopathic intestinal pseudo-obstruction. Gut. 2009;58(1):25-32. doi:10.1136/gut.2008.152512
- Sinagra E, Raimondo D, Gallo E, et al. Could JC virus be linked to chronic idiopathic intestinal pseudo-obstruction?. Clin J Gastroenterol. 2020;13(3):377-381. doi:10.1007/s12328-019-01069-4
- Arbour N, Day R, Newcombe J, Talbot PJ. Neuroinvasion by human respiratory coronaviruses. J Virol. 2000;74(19):8913-8921. doi:10.1128/jvi.74.19.8913-8921.2000
- Desforges M, Le Coupanec A, Brison E, Meessen-Pinard M, Talbot PJ. Neuroinvasive and neurotropic human respiratory coronaviruses: potential neurovirulent agents in humans. Adv Exp Med Biol. 2014;807:75-96. doi:10.1007/978-81-322-1777-0_6
- Xu J, Zhong S, Liu J, et al. Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Clin Infect Dis. 2005;41(8):1089-1096. doi:10.1086/444461
- Mao L, Jin H, Wang M, et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683-690. doi:10.1001/jamaneurol.2020.1127