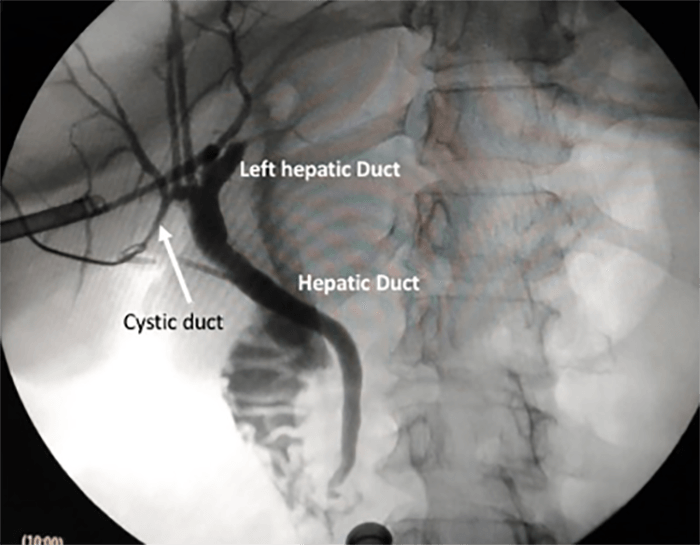
Nine months after initial presentation and treatment, the patient developed pain and a palpable mass in the left lower quadrant. A CT scan of the chest, abdomen and pelvis demonstrated a 2.5 x 2 cm mass in the anterior abdominal wall and biopsy suggested recurrent GCC. An octreotide scan failed to demonstrate uptake in the abdominal wall or other sites. The patient underwent excision of the mass that involved the rectus muscle and posterior rectus sheath. Pathology confirmed recurrent GCC. The patient declined systemic treatment at that time.
Two years after the initial presentation, he developed right lower quadrant pain with no other symptoms. CT scan of the abdomen demonstrated focal intestinal wall thickening and colonoscopy confirmed a mass at the anastomosis. Re-staging CT of the chest revealed no metastasis. He underwent resection of the involved colon and small bowel, again an R0 excision. Eighteen lymph nodes were negative for malignancy. Following surgical resection, he underwent adjuvant chemotherapy with folinic acid, fluorouracil, and oxaliplatin (FOLFOX).
A year after resection of the anastomotic recurrence, the patient developed an umbilical nodule and another mass in the right lower abdominal wall. These were histologically confirmed as GCC. Chest and abdominal CT scans showed no other metastases. He underwent palliative radiotherapy with a partial response.
A year later, he presented with an increase in the size of the abdominal wall lesions. Repeat biopsy affirmed the diagnosis of GCC. Repeat CT of the chest, abdomen and pelvis as well as whole body PET scan were negative for any other lesions. Genetic studies of the tumor revealed a BRAF V600E mutation, but no KRAS or NRAS mutations. He was started on a course of palliative chemotherapy with capecitabine and irinotecan, however, he failed to respond and had an interval increase in the size of the masses. Chemotherapy was therefore discontinued and the patient had resection of the masses with abdominal wall reconstruction with biological mesh. He is alive and disease free six years following initial presentation.
Discussion
Goblet cell carcinoids possess both neuroendocrine and glandular components and are thought to originate from single pluripotent stem cells.1,3,4 Almost exclusively found in the appendix, they are estimated to make up less than 5% of all appendiceal tumors.5 Their incidence has been extrapolated to between 0.01 to 0.05 per 100,000/year, and most are diagnosed in the fifth decade of life with no identified gender preferences.2,6
Immunohistochemical markers such as NSE and chromogranin A which are unique to neuroendocrine tumors are not always positive in GCCs.5,8 Another feature that distinguishes GCC from typical carcinoids, is an increase in circulating CEA levels.9 Our patient exhibited elevated CEA levels at the initial diagnosis and with each recurrence.
Unlike neuroendocrine tumors (NETs), Ki-67 index is not useful for grading GCCs.6,10 Tang’s classification has been found to be prognostic for GCC arising in the appendix.2 This is a pathologic classification which is based upon the proportion of goblet cell in the tumor. Our patient was assigned a Class B designation (Adenocarcinoma ex GCC, Signet Ring Cell Type) which signifies tumors that have a partial or almost total loss of goblet cell clusters and possess neoplastic cells that are individually arranged and infiltrate the appendix. Based on the study by Tang et al, three-year diseases specific survival (DSS) was 85% and 36% at five years. Tissue genetic studies have yielded different results.6,10 Generally, GCC tumors lack genetic mutations that are usually seen in colorectal adenocarcinoma, and they are usually negative for KRAS, SMAD4 and BRAF mutations.10,11 In our case, however, the tumor was positive for a BRAF mutation. The presence of KRAS/NRAS/BRAF mutations affect the choice of chemotherapy because tumors with such mutations are not responsive to cetuximab and panitumumab.12 Figure 5 shows a comparison of the features of colorectal cancer, appendiceal goblet cell carcinoid and our patient.
| | Colorectal cancer | Appendiceal
GCC | Our patient |
| Circulating Tumor Markers | | | |
| Chromogranin A | Normal | Variable (±) | Elevated |
| CEA | Elevated | Elevated | Elevated |
| Genetic Mutations | | | |
| KRAS | Variable (±) | Negative | Negative |
| BRAF | Variable (±) | Negative | Positive |
| Immunohistochemical staining | | | |
| NSE | Negative | Variable (±) | Positive |
| Chromogranin A | Negative | Variable (±) | Negative |
| Synaptophysin | Negative | Variable (±) | Positive |
Figure 5. Comparison of features of colorectal cancer, appendiceal goblet cell carcinoid and our patient with primary colonic goblet cell carcinoid (CEA: carcinoembryonic antigen; NSE:neuron specific enolase)
Appendiceal GCCs are managed following the guidelines for appendiceal adenocarcinomas. This aggressive approach is consistent with the proposed malignant nature of these tumors.11 However, because of its rarity, no guidelines exist for the evaluation or therapy of extra-appendiceal GCC. Our patient was managed in a multidisciplinary manner using guidelines for colorectal cancers. To accommodate its neuroendocrine nature, certain modifications were incorporated into the evaluation algorithm including serial measurement of chromogranin A and CEA. Octreotide scans were also performed but as others have found, their utility was minimal.11
While surgical resection of GCC adheres to established oncologic principles in terms of margins and harvesting lymph nodes, choice of chemotherapy is challenging. For appendiceal GCC, FOLFOX regimen has commonly been used in keeping with staging of appendiceal GCC similarly to colorectal adenocarcinoma but response is variable.2,6 The use of hyperthermic intraperitoneal chemotherapy (HIPEC) has been considered for patients with peritoneal disease and has been suggested to increase median survival.2,6 Our patient had an umbilical metastatic nodule and metastasis to the abdominal wall musculature, and he was therefore not amenable to HIPEC therapy.
Conclusion
Goblet cell carcinoids are usually found in the appendix, however, extra-appendiceal primary tumors can occur. A diagnosis of primary extra-appendiceal GCC requires histologic examination of the appendix. No guidelines exist for the management of colonic GCC. However, while our patient suffered recurrences and abdominal wall metastasis, our treatment regimen may have had an effect on the growth of the tumor, as the patient remains alive and disease free at six years.
Lessons Learned
In the setting of extra-appendiceal GCC the appendix should be examined histologically to distinguish primary extra-appendiceal GCC (extremely rare) from metastatic appendiceal GCC.
Authors
Thomas Murickan, MD
Michigan State University College of Human Medicine
Department of Surgery
East Lansing, MI
Edward Sparrow Hospital
Department of Pathology
Lansing, MI
Chibueze Onyemkpa, MD
Michigan State University College of Human Medicine
Department of Surgery
East Lansing, MI
Edward Sparrow Hospital
Department of Pathology
Lansing, MI
Christopher Flynn, MD
Edward Sparrow Hospital
Department of Pathology
Lansing, MI
Srinivas Kavuturu, MD, FACS
Michigan State University College of Human Medicine
Department of Surgery
East Lansing, MI
Correspondence
Chibueze Onyemkpa, MD
Department of Surgery
Michigan State College of Human Medicine
4660 Hagadorn Road, Suite 600
East Lansing, MI 48823
Email: Chibueze.Onyemkpa@hc.msu.edu
Phone: 347-567 9273
Disclosures
The authors have no conflicts of interest.
References
- Byrn JC, Wang J-L, Divino CM, Nguyen SQ, Warner RR. Management of goblet cell carcinoid. Journal of Surgical Oncology. 2006;94(5):396-402
- Olsen IH, Holt N, Langer SW, et al. Goblet Cell Carcinoids: Characteristics of a Danish Cohort of 83 Patients. Plos One. 2015;10(2)
- Gui X, Qin L, Gao Z-H, Falck V, Harpaz N. Goblet cell carcinoids at extraappendiceal locations of gastrointestinal tract: An underrecognized diagnostic pitfall. Journal of Surgical Oncology. 2011;103(8):790-795.
- Kang Y, Choi J-W, Kim Y, Oh HE, Lee J-H, Kim Y-S. Goblet Cell Carcinoid of the Rectum in a Patient with Neurofibromatosis Type 1. Journal of Pathology and Translational Medicine. 2016;50(6):482-485.
- Mitra B, Pal M, Paul B, Saha TN, Maiti A. Goblet cell carcinoid of appendix: A rare case with literature review. International Journal of Surgery Case Reports. 2013;4(3):334-337.
- Pape U-F, Perren A, Niederle B, et al. ENETS Consensus Guidelines for the Management of Patients with Neuroendocrine Neoplasms from the Jejuno-Ileum and the Appendix Including Goblet Cell Carcinomas. Neuroendocrinology. 2012;95(2):135-156.
- Tang LH, Shia J, Soslow RA, et al. Pathologic Classification and Clinical Behavior of the Spectrum of Goblet Cell Carcinoid Tumors of the Appendix. The American Journal of Surgical Pathology. 2008;32(10):1429-1443.
- Roy P. Goblet cell carcinoid tumors of the appendix: An overview. World Journal of Gastrointestinal Oncology. 2010;2(6):251.
- Eeden SV, Offerhaus GJA, Hart AAM, et al. Goblet cell carcinoid of the appendix: a specific type of carcinoma. Histopathology. 2007;51(6):763-773.
- Wen KW, Hale G, Shafizadeh N, Hosseini M, Huang A, Kakar S. Appendiceal goblet cell carcinoid: common errors in staging and clinical interpretation with a proposal for an improved terminology. Human Pathology. 2017;65:187-193
- Shenoy S. Goblet cell carcinoids of the appendix: Tumor biology, mutations and management strategies. World Journal of Gastrointestinal Surgery. 2016;8(10):660.
- Pietrantonio F, Cremolini C, Petrelli F, et al. First-line anti-EGFR monoclonal antibodies in panRAS wild-type metastatic colorectal cancer: A systematic review and meta-analysis. Critical Reviews in Oncology/Hematology. 2015;96(1):156-166.