Abstract
Background
Lymphoceles are a common complication of kidney allotransplantation, reported in up to 26% of kidney transplant recipients. They occur secondary to transection of lymphatic vessels during dissection of the iliac vasculature and can cause mass effect compression of the transplanted kidney, ureter, or vessels, compromising allograft function. Interventions for postoperative lymphocele include percutaneous aspiration, laparoscopic, or open peritoneal fenestration. Percutaneous drainage or peritoneal fenestration are associated with complications including infection, bleeding, bladder or ureter injury, and postoperative hernia, as well as, a recurrence rate ranging from 16 to 50%. Alternatively, ethiodized oil lymphangiography has been described for diagnosis and treatment of various postoperative lymphatic leakages, including chylothorax, chylous ascites, or lymphatic fistulas, with success rates reported up to 94%.
Summary
A 21-year-old male with end-stage renal disease secondary to focal segmental glomerulosclerosis in the setting of Sjögren’s syndrome underwent an initially uneventful deceased donor kidney transplant. Postoperative Jackson-Pratt drain output was persistently elevated, with fluid appearance consistent with a lymphocele. He underwent lymphangiography without embolization on postoperative day eight, which confirmed a lymphatic leakage site in the right pelvic retroperitoneum. Following the procedure his drain output decreased, fluid appeared serous, and his drain was removed.
Conclusion
Recurrence rate of postoperative lymphocele after kidney transplant is reported as high as 50% after percutaneous drainage and 16% after laparoscopic or open fenestration. These interventions are also associated with complications including infection, bladder or ureter injury, bleeding, or postoperative hernia. We present a case of a patient with posttransplant lymphocele managed using lymphangiography alone. This technique is associated with few post-procedure complications in comparison to more invasive approaches. Further research is needed on the role of lymphangiography in posttransplant lymphocele.
Key Words
lymphangiography, lymphocele, kidney transplant
Case Description
Postoperative fluid collections around kidney transplants have a number of etiologies, including hematoma, seroma, urinoma, or lymphocele. These can be differentiated by the characteristic and volume of the fluid as well as with laboratory studies. Postoperative lymphoceles occur in up to 26% of kidney transplant patients and pose a management dilemma to surgeons.1 Lymphoceles can occur secondary to transection of recipient lymphatic vessels during dissection of the iliac vasculature, as Tiong et al, demonstrated that minimizing dissection during exposure of the vessels led to decreased rates of lymphoceles.2 Alternatively, a lymphatic leak can occur due to dissection of donor lymphatics at the renal hilum.3 Finally, various factors such as delayed graft function, use of mTOR inhibitors, or high dose steroids have correlated with higher rates of postoperative lymphatic leaks.2,3 Strategies to minimize risk of lymphocele include prophylactic peritoneal fenestration at the time of transplant, meticulous dissection of donor and recipient vessels, as well as novel techniques such as application of hemostatic or sealant agents, or lower limb compression therapy.
While small lymphoceles can often resolve without intervention, larger lymphoceles can result in compression of the transplanted kidney, ureter, or renal vessels, with associated morbidity. Initial conservative management can be attempted with a low-fat diet, however, prolonged or severe compression carries the risk of allograft loss secondary to Page kidney phenomenon, thereby necessitating intervention for decompression.4 A number of procedures can be performed to control the lymphatic leak and alleviate these risks. These procedures range from less invasive simple aspiration and sclerotherapy, to laparoscopic or open fenestration of the peritoneal cavity to allow drainage.5 However, each intervention carries a risk of recurrence, as well as other possible complications.
Simple aspiration of the lymphocele does not address the lymphatic leak, and therefore carries a recurrence rate reported as high as 50%, necessitating further interventions.6 Aspiration can increase the risk of post-procedure infections, as can sclerotherapy or drain placement. Sclerosing therapy frequently requires repeated procedures, prolonging the time to definitive resolution. Peritoneal fenestration is the current gold standard for definitive management of posttransplant lymphoceles, however, this intervention still has a 16% or greater recurrence rate. Furthermore, this more invasive approach to lymphocele management requires general anesthesia and has higher morbidity rates with more severe complications, which plays a particularly important role the already comorbid transplant patient population. Both open and laparoscopic fenestration can cause ureteral or bladder injury, bleeding, or postoperative hernias.5,6
Lymphatic leakage is a common complication after pelvic surgery involving lymphadenectomy. Interventional lymphangiography has been described for both diagnosis and treatment of lymphatic leakages after such operative procedures.7 The ethiodized oil substrate used in lymphangiography causes a granulomatous reaction, which can lead to resolution of the lymphatic leak alone. Additionally, lymphangiography can be performed in conjunction with embolization, either at the time of the initial procedure, or on repeat procedure, for definitive treatment of the leak.8 In general, lymphangiography is diagnostic for leak in 64 to 78% of cases, with expanding applications for therapeutic interventions.12 Matsumoto et al report a case series of eight patients with postoperative chyle leak who were successfully treated with lymphangiography after failing conservative management.9 Additionally, Baek et al demonstrated the therapeutic potential of lymphangiography in five patients with pelvic lymphocele after gynecologic surgery.10 There are no reports of lymphangiography for management of postoperative lymphocele in kidney transplant patients.
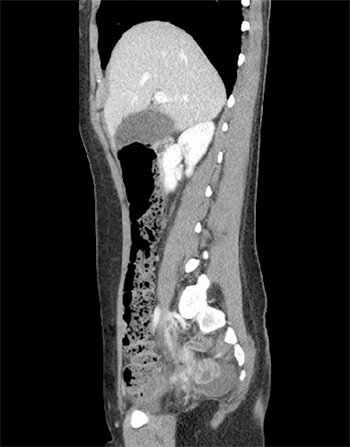
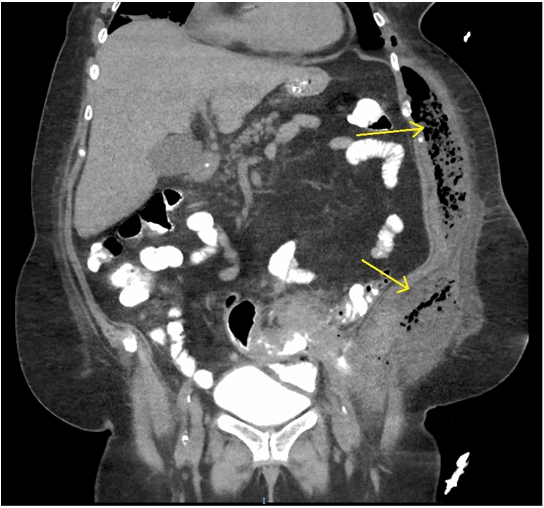
A 21-year-old African American male with end-stage renal disease secondary to focal segmental glomerulosclerosis in the setting of Sjögren’s syndrome underwent deceased-donor kidney transplant with thymoglobulin induction. During the iliac dissection, there were no enlarged lymphatic vessels noted, and care was taken to avoid transection of identified lymphatic channels. A Jackson-Pratt drain was placed in the right iliac fossa at the end of the case, at the discretion of the attending surgeon. His immediate postoperative course was uneventful, however, he presented to postoperative follow-up appointments complaining of persistently high Jackson Pratt drain output (500-600cc/day). The fluid was sent for creatinine to rule out a urinoma. Given concern for lymphocele, he underwent lymphangiography without embolization on postoperative day eight with the interventional radiology team. At this time, 10cc of ethiodized oil was injected into a right inguinal lymph node, and intermittent fluoroscopy showed a single area of fluid extravasation consistent with a lymphatic leak in the right pelvic retroperitoneum (Figure 1). After the procedure his drain fluid cleared, becoming serous appearing, and output decreased to less than 50cc/day. His drain was ultimately removed on post-procedure day 15 (postoperative day 23). Subsequent follow-up appointments demonstrate preserved allograft function with no evidence of lymphocele recurrence through 12 months postoperatively.